Scaptomyza flava
Scaptomyza flava is an herbivorous fly species in the family Drosophilidae. The fly is brown in color and approximately 2.5 mm in length.[1][2] The larvae of the fly is a known pest of plants in the family Brassicaceae, the mustard plants. They are present in Europe and New Zealand.[3][4][5] It is unique among Drospophilidae because it is one of few herbivorous species in the family.[6]
| Scaptomyza flava | |
|---|---|
 | |
| Scaptomyza flava | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | S. flava |
| Binomial name | |
| Scaptomyza flava Fallen, 1823 | |
Distribution
Scaptomyza flava is commonly found in Europe and only recently has been discovered in New Zealand.[2][7] This is in contrast to other species in the genus Scaptomyza, which have been found on all continents except Antarctica.[8]
Phylogeny
Scaptomyza flava belongs to the order Diptera, the family Drosophilidae, and the genus Scaptomyza along with approximately 270 other species of flies.[9] The genus Scaptomyza contains various herbivorous species who all have leaf-mining larvae in common.[10]Flava is Latin for golden or yellow.[11]
Originally the fly was classified as Drosophila flava when first described in 1823, but the later name change to Scaptomyza flava reflects a more modern understanding of the fly's genetic and evolutionary relationship with similar species. The species has also previously been referred to as Scaptomyza apicalis and Scaptomyza flaveola.[10] The most closely related species is Scaptomyza nigra.[12]
Life history
Life cycle
In captivity, the duration of the Scaptomyza flava life cycle is approximately three weeks, with an average egg to adult growth period of around 20.52 days . Eggs are laid in feeding punctures introduced to the host plant by the female. Larvae can be expected to hatch somewhere around 48 hours after the female deposits the eggs in the puncture. Once hatched, the larvae feed on the leaves as they make their way through the veins toward the petiole, then either double back on their path, or go to a new leaf. Most individuals remain in the same plant through multiple stages of development.[10] The larvae then either remain in the leaves or leave the host to pupate.[10][5]


Mating
Scaptomyza flava participate in anisogamous mating. When males are exposed to females in a laboratory setting, they take two to three minutes to begin mating rituals. Generally, a male approaches a female and in a display of courtship flaps his wings and touches her body with his front legs. Females then become stationary as the male mounts her, and copulation ensues. Uninterrupted copulation typically lasts around twenty minutes. After mating, the males vibrate their wings and leave the female behind, showing no further interest.[5]
Mating displays differed in frequency between mated and virgin males. Mated males are significantly more likely to practice courtship behavior and successfully mate than virgin flies. This suggests that male Scaptomyza flava are capable of learning from previous experience. This behavior has also been reported in the distantly-related male fruit fly, who are also included in the family Drosophilidae.[5] In the lab, scientists found that 90% of flies will mate once or twice, with a much smaller percentage mating three or more times.[13] Scientists have suggested that the mating patterns of S. flava are linked to their circadian rhythm.[13]
Oviposition and fertility
S. flava were shown to begin oviposition approximately 2.7 days after emergence, with peak oviposition happening somewhere between five and ten days post-emergence. Over a lifetime, the average female produced approximately 130 eggs, of which approximately 71 were fertilized.[13] Both oviposition and fertility peaked within the first five to ten days post-emergence, with approximately 50% of the viable eggs having been laid within the first 14 days from when the female began ovipositing.[13]
Food sources
Diet
Scaptomyza flava are considered parasitoids because they live in and consume various plants.
Larvae
Scaptomyza flava larvae are dependent on living plants as a food source, as opposed to other herbivorous insects that can digest decaying plant matter.[12] One benefit of this behavior is protection from pathogens, but this protection comes at the price of increased susceptibility to parasitoid attack.[10] Scaptomyza flava larvae live in and feed on plants in the family Brassicaceae, more commonly referred to as mustard plants.[7][10] Plants in the family Brassicaceae include arugula, broccoli, Brussel sprouts, bok choy, cauliflower, and turnips.[3] In New Zealand, the larvae are also known to infect gypsophila, otherwise known as baby's breath.[5]
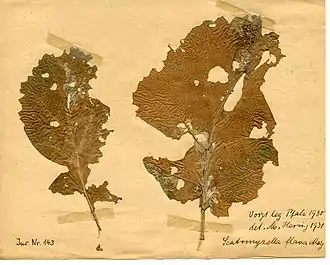
Adult
Adult females also feed on the same plants as their larvae. Instead of eating the plants from the inside out, however, they feed on the plant secretions induced by the punctures they make with their ovipositors.[10]
Evolution of herbivory
Herbivory is not unique to the genus Scaptomyza, occurring in many insects around the world through various evolutionary events. Specifically, herbivory in Scaptomyza is expected to have evolved between six and sixteen million years ago, with the most recent estimates placing this evolutionary event at approximately 13.5 million years ago.[6][12] The trait is associated with the split from the most recent common ancestor of Scaptomyza flava and Scaptomyza nigrita.[12]
Genetic analysis suggests herbivory evolved only once in the genus Scaptomyza. Other species in the family Drosophilidae have a strong affinity for sensing yeast, a trait that Scaptomyza flava does not possess. Scientists suggest that the loss of ability to sense yeast is associated with evolutionary events leading to the development of herbivory.[6]
Bypassing glucosinolates
The species Scaptomyza flava and Scaptomyza nigrita have specifically evolved the ability to partially metabolize glucosinolates, which are toxic chemicals synthesized by mustard plants as a defense mechanism. Other species in the family Drosophilidae are largely unable to overcome these toxins. Glucosinolates play a role in regulating stress-related genes in Scaptomyza flava, which is different from the specialized systems that other herbivores have developed to bypass the toxic effect of the chemical.[12]
Mutualism
Scaptomyza flava adult females and larvae inoculate their host plants with bacteria to aid in the feeding process. Specifically, Scaptomyza flava may introduce Pseudomonas syringae to the host plant, which damages the anti-herbivore defenses of the host plant. Both adult females and larvae are capable of acting as bacterial vectors. Scaptomyza flava larvae not only prefer to eat plants infected with Pseudomonas syringae, but have actually been shown to develop faster when feeding on infected plants.[14]
Interactions with humans
Scaptomyza flava feed on various agricultural staples, often destroying the plants they live in. While heavy infestations on various food crops can reduce yields, specific measures are not usually taken to contain the fly.[5]
References
- "Scaptomyza flava | NatureSpot". www.naturespot.org.uk. Retrieved 2019-09-30.
- "Scaptomyza (Scaptomyza) flava (Fallen, 1823) | Fauna Europaea". fauna-eu.org. Retrieved 2019-09-30.
- "List of plants in the family Brassicaceae". Encyclopedia Britannica. Retrieved 2019-09-30.
- "Scaptomyza (Scaptomyza) flava (Fallen, 1823) | Fauna Europaea". fauna-eu.org. Retrieved 2019-09-30.
- Martin, N. A. (January 2004). "History of an invader, Scaptomyza flava (Fallen, 1823) (Diptera: Drosophilidae)". New Zealand Journal of Zoology. 31 (1): 27–32. doi:10.1080/03014223.2004.9518356. ISSN 0301-4223.
- Goldman-Huertas, Benjamin; Mitchell, Robert F.; Lapoint, Richard T.; Faucher, Cécile P.; Hildebrand, John G.; Whiteman, Noah K. (2015-01-26). "Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet". Proceedings of the National Academy of Sciences. 112 (10): 3026–3031. doi:10.1073/pnas.1424656112. ISSN 0027-8424.
- Martin, N. A. (2004-01-01). "History of an invader, Scaptomyza flava (Fallen, 1823) (Diptera: Drosophilidae)". New Zealand Journal of Zoology. 31 (1): 27–32. doi:10.1080/03014223.2004.9518356. ISSN 0301-4223.
- Lapoint, Richard T.; O’Grady, Patrick M.; Whiteman, Noah K. (2013-10-01). "Diversification and dispersal of the Hawaiian Drosophilidae: The evolution of Scaptomyza" (PDF). Molecular Phylogenetics and Evolution. 69 (1): 95–108. doi:10.1016/j.ympev.2013.04.032. ISSN 1055-7903. PMID 23669011.
- "ADW: Scaptomyza: CLASSIFICATION". animaldiversity.org. Retrieved 2019-09-30.
- Whiteman, Noah K.; Groen, Simon C.; Chevasco, Daniela; Bear, Ashley; Beckwith, Noor; Gregory, T. Ryan; Denoux, Carine; Mammarella, Nicole; Ausubel, Frederick M.; Pierce, Naomi E. (March 2011). "Mining the plant-herbivore interface with a leafmining Drosophila of Arabidopsis". Molecular Ecology. 20 (5): 995–1014. doi:10.1111/j.1365-294X.2010.04901.x. ISSN 0962-1083. PMC 3062943. PMID 21073583.
- "Latin Definition for: flavus, flava, flavum (ID: 20735) - Latin Dictionary and Grammar Resources - Latdict". latin-dictionary.net. Retrieved 2019-10-02.
- Whiteman, Noah K.; Gloss, Andrew D.; Sackton, Timothy B.; Groen, Simon C.; Humphrey, Parris T.; Lapoint, Richard T.; Sønderby, Ida E.; Halkier, Barbara A.; Kocks, Christine; Ausubel, Frederick M.; Pierce, Naomi E. (2012). "Genes involved in the evolution of herbivory by a leaf-mining, Drosophilid fly". Genome Biology and Evolution. 4 (9): 900–916. doi:10.1093/gbe/evs063. ISSN 1759-6653. PMC 3516228. PMID 22813779.
- Shakeel, M.; He, X.Z.; Martin, N.A.; Hanan, A.; Wang, Q. (2009-08-01). "Diurnal periodicity of adult eclosion mating and oviposition of the european leafminer Scaptomyza flava (Falln) (Diptera Drosophilidae)". New Zealand Plant Protection. 62: 80–85. doi:10.30843/nzpp.2009.62.4789. ISSN 1179-352X.
- Groen, Simon C.; Humphrey, Parris T.; Chevasco, Daniela; Ausubel, Frederick M.; Pierce, Naomi E.; Whiteman, Noah K. (January 2016). "Pseudomonas syringae enhances herbivory by suppressing the reactive oxygen burst in Arabidopsis". Journal of Insect Physiology. 84: 90–102. doi:10.1016/j.jinsphys.2015.07.011. ISSN 1879-1611. PMC 4721946. PMID 26205072.