Selenite (ion)
The selenite anion is a selenium oxyanion with the chemical formula SeO2−
3.
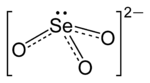
Structure of selenite
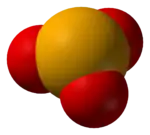
Space-filling model of selenite
A selenite (compound) is a compound that contains this ion.
In slightly acid conditions, the hydrogenselenite ion, HSeO−
3, is formed; in more acidic conditions selenous acid, H2SeO3, exists.
Most selenite salts can be formed by heating the relevant metal oxide with selenium dioxide, e.g.:
- Na2O + SeO2 → Na2SeO3.
See Category:Selenites for a list.
External links
| Wikimedia Commons has media related to Selenites. |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.