Sodium thiosulfate (medical use)
Sodium thiosulfate, also spelled sodium thiosulphate, is used as a medication to treat cyanide poisoning, pityriasis versicolor, and to decrease side effects from cisplatin.[1][2] For cyanide poisoning it is often used after the medication sodium nitrite and typically only recommended for severe cases.[1][3] It is either given by injection into a vein or applied to the skin.[1]
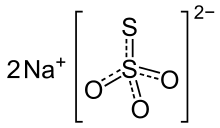 Sodium thiosulfate, structural formula | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | Na2O3S2 |
| Molar mass | 158.10 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Side effects may include vomiting, joint pain, mood changes, psychosis, and ringing in the ears.[2] Safety, however, has not been well studied.[4] It is unclear if use in pregnancy is safe for the baby.[2] Use at the same time in the same intravenous line as hydroxocobalamin is not recommended.[3] In cyanide poisoning sodium nitrite creates methemoglobinemia which removes cyanide from mitochondria.[3] Sodium thiosulfate then binds with cyanide creating the nontoxic thiocyanate.[3]
Sodium thiosulfate came into medical use for cyanide poisoning in the 1930s.[5] It is on the World Health Organization's List of Essential Medicines.[6] The cost in the United States per dose as of 2013 is about US$20 while together with sodium nitrite it costs US$110.[7]
Medical uses
The main use of sodium thiosulfate is in cyanide poisoning and pityriasis versicolor.[1]
Cyanide poisoning
Sodium thiocyanate is a classical antidote to cyanide poisoning,[8] For this purpose it is used after the medication sodium nitrite and typically only recommended for severe cases.[1][3] It is given by injection into a vein.[1]
In this use, sodium nitrite creates methemoglobinemia which removes cyanide from mitochondria.[3] Sodium thiosulfate then serves as a sulfur donor for the conversion of cyanide to the nontoxic thiocyanate, catalyzed by the enzyme rhodanase. The thiocyanate is then safely excreted in the urine.[3][9]
There are concerns that sodium thiosulfate may not have a fast enough onset of action to be very useful for this use without the additional use of other agents.[9]
In cases with both cyanide poisoning and carbon monoxide poisoning, sodium thiosulfate by itself is recommended.[10]
Hemodialysis
There is a small amount of evidence supporting the use of sodium thiosulfate to counteract calciphylaxis, the calcification of blood vessels that may occur in hemodialysis patients with end-stage kidney disease.[11][12]
However, it has been claimed that this treatment may cause severe metabolic acidosis in some patients.[13][14]
Sodium thiosulfate has been observed to help in the treatment of a rare systemic fibrosis condition caused by gadolinium-based contrast media in patients with kidney failure.[15]
The compound can also be used to measure the volume of extracellular body fluid and the renal glomerular filtration rate.[16]
Fungal infections of the skin
Foot baths of sodium thiosulfate are used for prophylaxis of ringworm. It is also used as a topical antifungal agent for tinea versicolor (pityriasis versicolor), possibly in combination with salicylic acid;[17][18] and for other fungal infections of the skin.[19]
Side effects
Side effects may include vomiting, joint pain, mood changes, psychosis, and ringing in the ears.[2] Safety; however, has not been well studied.[4] It is unclear if use in pregnancy is safe for the baby.[2] Use at the same time in the same intravensous line as hydroxocobalamin is not recommended.[3]
History
Sodium thiosulfate came into medical use for cyanide poisoning in the 1930s.[20]
References
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 66. hdl:10665/44053. ISBN 9789241547659.
- "Sodium thiosulfate Intravenous Advanced Patient Information - Drugs.com". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 15 January 2017.
- "Sodium Thiosulfate Solution for Injection - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 16 January 2017. Retrieved 15 January 2017.
- "Sodium Thiosulfate Injection - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 15 January 2017.
- Dart, Richard C. (2004). Medical Toxicology. Lippincott Williams & Wilkins. p. 172. ISBN 9780781728454. Archived from the original on 2017-01-16.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Gasco, L; Rosbolt, MB; Bebarta, VS (April 2013). "Insufficient stocking of cyanide antidotes in US hospitals that provide emergency care". Journal of Pharmacology & Pharmacotherapeutics. 4 (2): 95–102. doi:10.4103/0976-500x.110875. PMC 3669589. PMID 23761707.
- "Toxicity, Cyanide: Overview". eMedicine. Retrieved 2009-01-01.
- Hall AH, Dart R, Bogdan G (June 2007). "Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning?". Annals of Emergency Medicine. 49 (6): 806–13. doi:10.1016/j.annemergmed.2006.09.021. PMID 17098327.
- Baren JM (2008). Pediatric Emergency Medicine. Elsevier Health Sciences. p. 1018. ISBN 978-1416000877.
- Auriemma M, Carbone A, Di Liberato L, Cupaiolo A, Caponio C, De Simone C, Tulli A, Bonomini M, Amerio P (October 2011). "Treatment of cutaneous calciphylaxis with sodium thiosulfate: two case reports and a review of the literature". American Journal of Clinical Dermatology. 12 (5): 339–46. doi:10.2165/11587060-000000000-00000. PMID 21834598. S2CID 28366905.
- Cicone JS, Petronis JB, Embert CD, Spector DA (June 2004). "Successful treatment of calciphylaxis with intravenous sodium thiosulfate". American Journal of Kidney Diseases. 43 (6): 1104–8. doi:10.1053/j.ajkd.2004.03.018. PMID 15168392.
- Berns JS (24 April 2012). "Sodium Thiosulfate and Acidosis: A Puzzle for Readers". Medscape.
- Selk N, Rodby RA (Jan–Feb 2011). "Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy". Seminars in Dialysis. 24 (1): 85–8. doi:10.1111/j.1525-139X.2011.00848.x. PMID 21338397. S2CID 23196149.
- Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R (March 2007). "Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure--role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate". Clinical Journal of the American Society of Nephrology. 2 (2): 258–63. doi:10.2215/CJN.03250906. PMID 17699422.
- "Sodium thiosulfate" at Dorland's Medical Dictionary
- Sunenshine PJ, Schwartz RA, Janniger CK (September 1998). "Tinea versicolor". International Journal of Dermatology. 37 (9): 648–55. doi:10.1046/j.1365-4362.1998.00441.x. PMID 9762812. S2CID 75657768.
- Hu SW, Bigby M (October 2010). "Pityriasis versicolor: a systematic review of interventions". Archives of Dermatology. 146 (10): 1132–40. doi:10.1001/archdermatol.2010.259. PMID 20956647.
- Rezabek GH, Friedman AD (May 1992). "Superficial fungal infections of the skin. Diagnosis and current treatment recommendations". Drugs. 43 (5): 674–82. doi:10.2165/00003495-199243050-00004. PMID 1379146. S2CID 46982698.
- Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. p. 172. ISBN 9780781728454.
External links
- "Sodium thiosulfate". Drug Information Portal. U.S. National Library of Medicine.