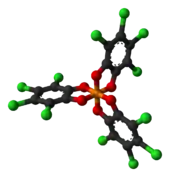
TRISPHAT
Tributylammonium TRISPHAT is an organic salt with the formula [(C
4H
9)
3NH+
][P(O
2C
6Cl
4)−
3]. The anion features phosphorus(V) bonded to three tetrachlorocatecholate (C
6Cl
4O2−
2) ligands. This anion can be resolved into the enantiomers, which are optically stable (the picture shows the Δ enantiomer).
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetrabutylammonium Phosphorus(V) tris(tetrachlorocatecholate)PHAT | |||
| Other names
Bu3NH+ PHAT− | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ECHA InfoCard | 100.164.647 | ||
CompTox Dashboard (EPA) |
|||
| |||
| Properties | |||
| [C16H36N][C18Cl12O6P] | |||
| Molar mass | 1011.06 | ||
| Appearance | colourless solid | ||
| CH2Cl2 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The TRISPHAT anion has been used as a chiral shift reagent for cations. It improves the resolution of 1H NMR spectra by forming diastereomeric ion pairs.
Preparation
The anion is prepared by treatment of phosphorus pentachloride with tetrachlorocatechol followed by a tertiary amine gives the anion:
- PCl5 + 3 C6Cl4(OH)2 → H[P(O2C6Cl4)3] + 5 HCl
H[P(O2C6Cl4)3] + Bu3N Bu3NH+ [P(O2C6Cl4)3]− Using a chiral amine, the anion can be readily resolved.[1]
References
- F. Favarger, C. Goujon-Ginglinger, D. Monchaud, J. Lacour "Large-Scale Synthesis and Resolution of TRISPHAT [Tris(tetrachlorobenzenediolato) Phosphate(V)] Anion" Journal of Organic Chemistry, 2004, volume 69, pp. 8521–8524, 2004. doi:10.1021/jo048641q.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.