Tabtoxin
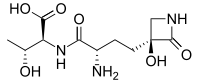
Tabtoxin, also known as wildfire toxin, is a simple monobactam phytotoxin produced by Pseudomonas syringae. It is the precursor to the antibiotic tabtoxinine β-lactam.[1] Tabtoxin is a monocyclic β-lactam produced by P. syringae pv. tabaci, coronafaciens, and garcae. Pseudomonas syringae pv. tabaci, the causal agent of the wildfire of tobacco, produces the phytotoxin tabtoxin. tabtoxin-producing bacterium, P. syringae BR2, causes a disease of bean (Phaseolus vulgaris) similar to tobacco wildfire. This organism is closely related to P. syringae pv. tabaci but cannot be classified in the pathovar tabaci because it is not pathogenic on tobacco. Tabtoxin has been shown to be a dipeptide precursor that must undergo hydrolysis by a peptidase to yield the biologically active form, tabtoxinine-p-lactam (TβL). Tabtoxin is required by BR2(R) for both chlorosis and lesion formation on bean. All mutations that affected tabtoxin production, whether spontaneous deletion or transposon induced, also affected lesion formation, and in all cases, restoration of tabtoxin production also restored pathogenic symptoms. Other factors may be required for BR2 to be pathogenic on bean, but apparently these are in addition to tabtoxin production.[2][3]
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3R)-2-((S)-2-Amino-4-((S)-3-hydroxy-2-oxoazetidin-3-yl)butanamido)-3-hydroxybutanoic acid | |
| Other names
N-[(2S)-2-Amino-4-[(3S)-3-hydroxy-2-oxo-3-azetidinyl]-1-oxobutyl]-L-threonine; (S)-γ-(3-Hydroxy-2-oxo-3-azetidinyl)-L-α-aminobutyryl-L-threonine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H19N3O6 | |
| Molar mass | 289.288 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Protein
Tabtoxin resistance protein (TTR) is an enzyme that catalyzes the acetylation of tabtoxin rendering tabtoxin-producing pathogens tolerant to their own phytotoxins. According to the structure based detoxification mechanism of TTR, three site-directed mutants Y141F, D130N and Y141F-D130N were constructed and overexpressed in E. coli. The products were then purified and their properties were analyzed by CD and DLS. The crystal structure of TTR complexed with its natural cofactor, acetyl coenzyme A (AcCoA), to 1.55 Å resolution. The binary complex forms a characteristic “V” shape for substrate binding and contains the four motifs conserved in the GCN5-related N-acetyltransferase (GNAT) superfamily, which also includes the histone acetyltransferases (HATs). There are reports that TTR possesses HAT activity and suggest an evolutionary relationship between TTR and other GNAT members. The dipeptide toxin contains tabtoxinine-β-lactam (TβL) linked by a peptide bond to threonine produced, the chlorosis-inducing activity occurs only after hydrolysis of the peptide bond by aminopeptidases of plant or bacterial origin. Cleavage of the peptide bond in tabtoxin releases TβL, the toxic moiety. TβL is located at the N terminus, and Thr is at the C terminus, resulting in TβL-Thr. TβL-Thr is hydrolyzed, and the resulting TβL irreversibly inhibits glutamine synthetase, causing characteristic chlorosis in plants. TβL is spontaneously isomerized to tabtoxinine-δ-lactam (TδL), and TblF did not recognize TδL as a substrate, yielding no TδL-Thr. Some characteristics were also provided by them, but only from the perspective of tabtoxin biosynthesis. βL-Thr is observed, but Thr-TβL, whose sequence is the reverse of that of tabtoxin, is not. The effects of carbon, nitrogen sources and amino acids on growth and tabtoxin production by pv. tabaci, were examined by varying the components of a defined basal medium, which contained the following nutrients per liter: sucrose (10 g), KNO3 (5 g), MgSO(4).7H2O (0.2 g), CaCl(2).2H2O (0.11 g), FeSO(4).7H2O (20 mg), NaH2PO(4).2H2O (0.9 g) and H2PO(4).3HO (1 g). Both growth and quantity of tabtoxin synthesized were significantly affected by carbon source, nitrogen source and amino acid supplements. Sorbitol, xylose and sucrose proved to be the best carbon sources for tabtoxin production. Specific toxin production was very low using glucose as a single carbohydrate source, although bacterial growth was well supported by glucose. Amount and type of nitrogen sources (NH4Cl or KNO3) affected the growth of pv. tabaci and quantities of tabtoxin produced. Nitrate is the best of these two forms of nitrogen for production of tabtoxin.[4][2]
Biosynthesis and Regulation
The biosynthetic precursors of tabtoxin were identified by the incorporation of 13C-labeled compounds and shown to consist of L-threonine and L-aspartate for the side chain and pyruvic acid and the methyl group of L-methionine for the β-lactam moiety. A biosynthetic model for the formation of TβL resembles that of lysine, where the first dedicated step is the DapA-catalyzed condensation of aspartic acid semialdehyde with pyruvate to form L-2,3-dihydropicolinate (DHDPA). Tabtoxin biosynthesis branches off from the lysine biosynthetic pathway before the formation of diaminopimelate (DAP). TabA is a gene, which is essential for tabtoxin production. The discovery of this gene provided the first experimental data to support the hypotheses that the precursors for tabtoxin originate from the lysine biosynthetic pathway. The deduced amino acid sequence of tabA showed significant relatedness to lysA, which encodes DAP decarboxylase in bacteria. Although tabA was not required for lysine biosynthesis, the deduced product of a tabB, also located in the TβL biosynthetic region, showed relatedness todapD, a gene encoding THDPA succinyl-CoA succinyltransferase (THDPA-ST). DapB is essential for both lysine and tabtoxin biosynthesis and THDPA may be an intermediate in both pathways. Three genes have been characterized in the 31-kb region which contains all genes necessary for TβL synthesis and tabtoxin resistance: tabA, tabB, and tblA. Although there is no obvious relationship between TblA and known polypeptides, TabA has significant sequence homology to LysA from E. coliand P. aeruginosa whereas TabB shows relatedness to DapD. Some progress has been made on elucidating factors that regulate tabtoxin biosynthesis in P. syringae. In a subsequent study, zinc was shown to be required for the aminopeptidase activity, which hydrolyzes tabtoxin to release TβL.[5]
References
- Kinscherf TG, Coleman RH, Barta TM, Willis DK (July 1991). "Cloning and expression of the tabtoxin biosynthetic region from Pseudomonas syringae". J. Bacteriol. 173 (13): 4124–32. doi:10.1128/jb.173.13.4124-4132.1991. PMC 208062. PMID 1648077.
- Arai, Toshinobu; Arimura, Yasuhiro; Ishikura, Shun; Kino, Kuniki (15 August 2013). "l-Amino Acid Ligase from Pseudomonas syringae Producing Tabtoxin Can Be Used for Enzymatic Synthesis of Various Functional Peptides". Appl. Environ. Microbiol. 79 (16): 5023–5029. doi:10.1128/AEM.01003-13. PMC 3754701. PMID 23770908.
- Kinscherf, T. G.; Coleman, R. H.; Barta, T. M.; Willis, D. K. (1 July 1991). "Cloning and expression of the tabtoxin biosynthetic region from Pseudomonas syringae". Journal of Bacteriology. 173 (13): 4124–4132. doi:10.1128/jb.173.13.4124-4132.1991. PMC 208062. PMID 1648077.
- Rao, Yi Ding, Shentao Li, Xiaofeng Li, Fei Sun, Jinyuan Liu, Nanming Zhao and Zihe (31 May 2003). "Site-Directed Mutagenesis and Preliminary X-Ray Crystallographic Studies of the Tabtoxin Resistance Protein". Protein & Peptide Letters. 10 (3): 255–63. doi:10.2174/0929866033478924. PMID 12871145.
- Bender, Carol L.; Alarcón-Chaidez, Francisco; Gross, Dennis C. (1 June 1999). "Pseudomonas syringae Phytotoxins: Mode of Action, Regulation, and Biosynthesis by Peptide and Polyketide Synthetases". Microbiol. Mol. Biol. Rev. 63 (2): 266–292. doi:10.1128/MMBR.63.2.266-292.1999. PMC 98966. PMID 10357851.
- http://aem.asm.org/content/79/16/5023.long
- He, Hongzhen; Ding, Yi; Bartlam, Mark; Sun, Fei; Le, Yi; Qin, Xincheng; Tang, Hong; Zhang, Rongguang; Joachimiak, Andrzej; Liu, Jinyuan; Zhao, Nanming; Rao, Zihe (2003). "Crystal Structure of Tabtoxin Resistance Protein Complexed with Acetyl Coenzyme A Reveals the Mechanism for β-Lactam Acetylation". Journal of Molecular Biology. 325 (5): 1019–1030. doi:10.1016/S0022-2836(02)01284-6. PMID 12527305.