Tait equation
In fluid mechanics, the Tait equation is an equation of state, used to relate liquid density to pressure. The equation was originally published by Peter Guthrie Tait in 1888 in the form[1]
where is the reference pressure (taken to be 1 atmosphere), is the current pressure, is the volume of fresh water at the reference pressure, is the volume at the current pressure, and are experimentally determined parameters.
Popular form of the Tait equation
Around 1895,[1] the original isothermal Tait equation was replaced by Tammann with an equation of the form
The temperature-dependent version of the above equation is popularly known as the Tait equation and is commonly written as[2]
or in the integrated form
where
- is the compressibility of the substance (often, water) (in units of bar−1 or Pa)
- is the specific volume of the substance (in units of ml/g or m3/kg)
- is the specific volume at = 1 bar
- and are functions of temperature that are independent of pressure[2]
Pressure formula
The expression for the pressure in terms of the specific volume is
Bulk modulus formula
The tangent bulk modulus at pressure is given by
Murnaghan-Tait equation of state
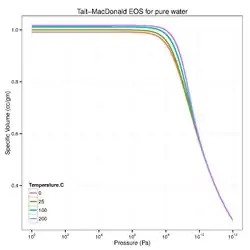
Another popular isothermal equation of state that goes by the name "Tait equation"[3][4] is the Murnaghan model[5] which is sometimes expressed as
where is the specific volume at pressure , is the specific volume at pressure , is the bulk modulus at , and is a material parameter.
Pressure formula
This equation, in pressure form, can be written as
where are mass densities at , respectively. For pure water, typical parameters are = 101,325 Pa, = 1000 kg/cu.m, = 2.15 GPa, and = 7.15.
Note that this form of the Tate equation of state is identical to that of the Murnaghan equation of state.
Bulk modulus formula
The tangent bulk modulus predicted by the MacDonald-Tait model is
Tumlirz-Tammann-Tait equation of state
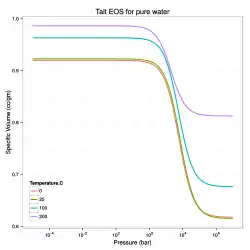
A related equation of state that can be used to model liquids is the Tumlirz equation (sometimes called the Tammann equation and originally proposed by Tumlirz in 1909 and Tammann in 1911 for pure water).[1][6] This relation has the form
where is the specific volume, is the pressure, is the salinity, is the temperature, and is the specific volume when , and are parameters that can be fit to experimental data.
The Tumlirz-Tammann version of the Tait equation for fresh water, i.e., when , is
For pure water, the temperature-dependence of are:[6]
In the above fits, the temperature is in degrees Celsius, is in bars, is in cc/gm, and is in bars-cc/gm.
Pressure formula
The inverse Tumlirz-Tammann-Tait relation for the pressure as a function of specific volume is
Bulk modulus formula
The Tumlirz-Tammann-Tait formula for the instantaneous tangent bulk modulus of pure water is a quadratic function of (for an alternative see [1])
See also
References
- Hayward, A. T. J. (1967). Compressibility equations for liquids: a comparative study. British Journal of Applied Physics, 18(7), 965. http://mitran-lab.amath.unc.edu:8081/subversion/Lithotripsy/MultiphysicsFocusing/biblio/TaitEquationOfState/Hayward_CompressEqnsLiquidsComparative1967.pdf
- Li, Yuan-Hui (15 May 1967). "Equation of State of Water and Sea Water" (PDF). Journal of Geophysical Research. Palisades, New York. 72 (10): 2665. Bibcode:1967JGR....72.2665L. doi:10.1029/JZ072i010p02665.
- Thompson, P. A., & Beavers, G. S. (1972). Compressible-fluid dynamics. Journal of Applied Mechanics, 39, 366.
- Kedrinskiy, V. K. (2006). Hydrodynamics of Explosion: experiments and models. Springer Science & Business Media.
- Macdonald, J. R. (1966). Some simple isothermal equations of state. Reviews of Modern Physics, 38(4), 669.
- Fisher, F. H., and O. E. Dial Jr. Equation of state of pure water and sea water. No. MPL-U-99/67. SCRIPPS INSTITUTION OF OCEANOGRAPHY LA JOLLA CA MARINE PHYSICAL LAB, 1975. http://www.dtic.mil/dtic/tr/fulltext/u2/a017775.pdf