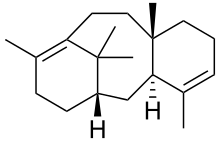
Taxadiene
Taxadiene (taxa-4,11-diene) is a diterpene. Taxadiene is the first committed intermediate in the synthesis of taxol.[1] Six hydroxylation reactions, and a few others, are needed to convert taxadiene to baccatin III.
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Taxa-4,11-diene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H32 | |
| Molar mass | 272.476 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Enzymatically, taxadiene is produced from geranylgeranyl pyrophosphate by taxadiene synthase. A biochemical gram-scale production of taxadiene has been reported in 2010 using genetically engineered Escherichia coli.[2]
References
- Lin, Xiaoyan; Hezari, Mehri; Koepp, Alfred E.; Floss, Heinz G.; Croteau, Rodney (1996). "Mechanism of Taxadiene Synthase, a Diterpene Cyclase That Catalyzes the First Step of Taxol Biosynthesis in Pacific Yew†". Biochemistry. 35 (9): 2968–77. doi:10.1021/bi9526239. PMID 8608134.
- Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, et al. (2010). "Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli" (PDF). Science. 330 (6000): 70–4. doi:10.1126/science.1191652. PMC 3034138. PMID 20929806.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.