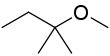
tert-Amyl methyl ether
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha.[4] It has an ethereous odor.[1] Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.[5]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Methoxy-2-methylbutane | |||
| Other names
tertiary-Amyl methyl ether; TAME; Methoxypentane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | TAME | ||
| ChemSpider | |||
| ECHA InfoCard | 100.012.374 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H14O | |||
| Molar mass | 102.177 g·mol−1 | ||
| Appearance | Clear, colorless liquid | ||
| Density | 0.76-0.78 g/mL[3] | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Boiling point | 86.3 °C (187.3 °F; 359.4 K) | ||
| 10.71 g/L at 20 °C | |||
Refractive index (nD) |
1.3896 | ||
| Hazards | |||
| Flash point | −11 °C (12 °F; 262 K) | ||
| 430 °C (806 °F; 703 K) | |||
| Explosive limits | 1.0-7.1% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Uses
TAME is mostly used as an oxygenate to gasoline. It is added for three reasons: to increase octane enhancement, to replace banned tetraethyl lead, and to raise the oxygen content in gasoline. It is known that TAME in fuel reduces exhaust emissions of some volatile organic compounds.[1]
TAME is also used as a solvent in organic synthesis as a more environmentally friendly alternative to some of the classic ether solvents.[4] It is characterized by a high boiling point (86 °C) and a low freezing point (−80 °C), allowing a wide range of reaction temperatures. TAME can be used as a safe reaction medium (e.g. condensation reactions, coupling reactions, such as Grignard reactions and Suzuki reactions, as well as metal hydride reductions) and as an extraction solvent to replace dichloromethane, aromatics, and other ethers.[6]
Toxicity
In an animal toxicology study, inhalation of high concentrations of TAME (4000 ppm) caused central nervous system depression, leading to death in most cases.[7]
References
- "tert-AMYL METHYL ETHER (1,1-DIMETHYLPROPYL METHYL ETHER)". chemicalland21.com. Retrieved 2009-10-20.
- National Industrial Chemicals Notification and Assessment Scheme (2001). "t-Amyl methyl ether (TAME)" (PDF). Full Public Reports. Retrieved 2009-10-20.
- "tert-Amyl methyl ether". Sigma-Aldrich.
- Prat, Denis; Wells, Andy; Hayler, John; Sneddon, Helen; McElroy, C. Robert; Abou-Shehada, Sarah; Dunn, Peter J. (2015-12-21). "CHEM21 selection guide of classical- and less classical-solvents". Green Chem. 18 (1): 288–296. doi:10.1039/c5gc01008j. ISSN 1463-9270.
- Diaz, Arthur F.; Drogos, Donna L. (2001-11-06). Oxygenates in Gasoline. ACS Symposium Series. 799. American Chemical Society. pp. 138–152. doi:10.1021/bk-2002-0799.ch010. ISBN 978-0841237605.
- Author. "INEOS Oligomers Products". www.ineos.com. Retrieved 2017-10-30.
- White, Russell D.; Daughtrey, Wayne C.; Wells, Mike S. (December 1995). "Health effects of inhaled tertiary amyl methyl ether and ethyl tertiary butyl ether". Toxicology Letters. 82–83: 719–724. doi:10.1016/0378-4274(95)03590-7. PMID 8597132.