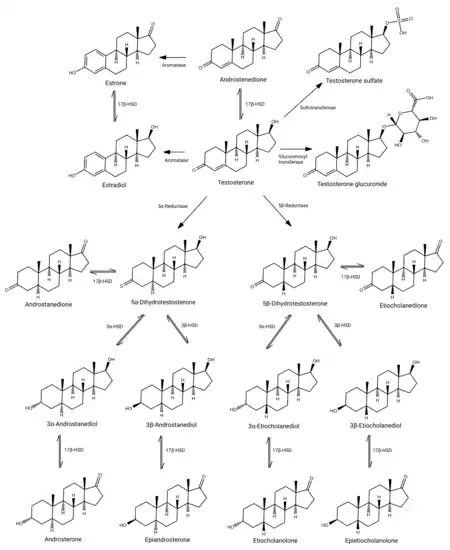
Testosterone
Testosterone is the primary sex hormone and anabolic steroid in males.[3] In male humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristics such as increased muscle and bone mass, and the growth of body hair.[4] In addition, testosterone is involved in health and well-being,[5] and the prevention of osteoporosis.[6] Insufficient levels of testosterone in men may lead to abnormalities including frailty and bone loss.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
17β-Hydroxyandrost-4-en-3-one | |
| Systematic IUPAC name
(8R,9S,10R,13S,14S,17S)-17-Hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one | |
| Other names
Androst-4-en-17β-ol-3-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.336 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H28O2 | |
| Molar mass | 288.431 g·mol−1 |
| Melting point | 151.0 °C (303.8 °F; 424.1 K)[1] |
| Pharmacology | |
| G03BA03 (WHO) | |
| License data | |
| Transdermal (gel, cream, solution, patch), by mouth (as testosterone undecanoate), in the cheek, intranasal (gel), intramuscular injection (as esters), subcutaneous pellets | |
| Pharmacokinetics: | |
| Oral: very low (due to extensive first pass metabolism) | |
| 97.0–99.5% (to SHBG and albumin)[2] | |
| Liver (mainly reduction and conjugation) | |
| 2–4 hours | |
| Urine (90%), feces (6%) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Testosterone is a steroid from the androstane class containing a keto and hydroxyl groups at positions three and seventeen respectively. It is biosynthesized in several steps from cholesterol and is converted in the liver to inactive metabolites.[7] It exerts its action through binding to and activation of the androgen receptor.[7] In humans and most other vertebrates, testosterone is secreted primarily by the testicles of males and, to a lesser extent, the ovaries of females. On average, in adult males, levels of testosterone are about seven to eight times as great as in adult females.[8] As the metabolism of testosterone in males is more pronounced, the daily production is about 20 times greater in men.[9][10] Females are also more sensitive to the hormone.[11]
In addition to its role as a natural hormone, testosterone is used as a medication in the treatment of hypogonadism in men and breast cancer in women.[12] Since testosterone levels decrease as men age, testosterone is sometimes used in older men to counteract this deficiency. It is also used as part of transgender hormone therapy for transgender men and illicitly to enhance physique and performance, for instance in athletes.[13]
Biological effects
In general, androgens such as testosterone promote protein synthesis and thus growth of tissues with androgen receptors.[14] Testosterone can be described as having virilising and anabolic effects (though these categorical descriptions are somewhat arbitrary, as there is a great deal of mutual overlap between them).[15]
- Anabolic effects include growth of muscle mass and strength, increased bone density and strength, and stimulation of linear growth and bone maturation.
- Androgenic effects include maturation of the sex organs, particularly the penis and the formation of the scrotum in the fetus, and after birth (usually at puberty) a deepening of the voice, growth of facial hair (such as the beard) and axillary (underarm) hair. Many of these fall into the category of male secondary sex characteristics.
Testosterone effects can also be classified by the age of usual occurrence. For postnatal effects in both males and females, these are mostly dependent on the levels and duration of circulating free testosterone.
Before birth
Effects before birth are divided into two categories, classified in relation to the stages of development.
The first period occurs between 4 and 6 weeks of the gestation. Examples include genital virilisation such as midline fusion, phallic urethra, scrotal thinning and rugation, and phallic enlargement; although the role of testosterone is far smaller than that of dihydrotestosterone. There is also development of the prostate gland and seminal vesicles.
During the second trimester, androgen level is associated with sex formation.[16] Specifically, testosterone, along with anti-Müllerian hormone (AMH) promote growth of the Wolffian duct and degeneration of the Müllerian duct respectively.[17] This period affects the femininization or masculinization of the fetus and can be a better predictor of feminine or masculine behaviours such as sex typed behaviour than an adult's own levels. Prenatal androgens apparently influence interests and engagement in gendered activities and have moderate effects on spatial abilities.[18] Among women with CAH, a male-typical play in childhood correlated with reduced satisfaction with the female gender and reduced heterosexual interest in adulthood.[19]
Early infancy
Early infancy androgen effects are the least understood. In the first weeks of life for male infants, testosterone levels rise. The levels remain in a pubertal range for a few months, but usually reach the barely detectable levels of childhood by 4–7 months of age.[20][21] The function of this rise in humans is unknown. It has been theorized that brain masculinization is occurring since no significant changes have been identified in other parts of the body.[22] The male brain is masculinized by the aromatization of testosterone into estrogen, which crosses the blood–brain barrier and enters the male brain, whereas female fetuses have α-fetoprotein, which binds the estrogen so that female brains are not affected.[23]
Before puberty
Before puberty effects of rising androgen levels occur in both boys and girls. These include adult-type body odor, increased oiliness of skin and hair, acne, pubarche (appearance of pubic hair), axillary hair (armpit hair), growth spurt, accelerated bone maturation, and facial hair.[24]
Pubertal
Pubertal effects begin to occur when androgen has been higher than normal adult female levels for months or years. In males, these are usual late pubertal effects, and occur in women after prolonged periods of heightened levels of free testosterone in the blood. The effects include:[24][25]
Growth of spermatogenic tissue in testicles, male fertility, penis or clitoris enlargement, increased libido and frequency of erection or clitoral engorgement occurs. Growth of jaw, brow, chin, and nose and remodeling of facial bone contours, in conjunction with human growth hormone occurs.[26] Completion of bone maturation and termination of growth. This occurs indirectly via estradiol metabolites and hence more gradually in men than women. Increased muscle strength and mass, shoulders become broader and rib cage expands, deepening of voice, growth of the Adam's apple. Enlargement of sebaceous glands. This might cause acne, subcutaneous fat in face decreases. Pubic hair extends to thighs and up toward umbilicus, development of facial hair (sideburns, beard, moustache), loss of scalp hair (androgenetic alopecia), increase in chest hair, periareolar hair, perianal hair, leg hair, armpit hair.
Adult
Testosterone is necessary for normal sperm development. It activates genes in Sertoli cells, which promote differentiation of spermatogonia. It regulates acute HPA (hypothalamic–pituitary–adrenal axis) response under dominance challenge.[27] Androgen including testosterone enhances muscle growth. Testosterone also regulates the population of thromboxane A2 receptors on megakaryocytes and platelets and hence platelet aggregation in humans.[28][29]
Adult testosterone effects are more clearly demonstrable in males than in females, but are likely important to both sexes. Some of these effects may decline as testosterone levels might decrease in the later decades of adult life.[30]
Health risks
Testosterone does not appear to increase the risk of developing prostate cancer. In people who have undergone testosterone deprivation therapy, testosterone increases beyond the castrate level have been shown to increase the rate of spread of an existing prostate cancer.[31][32][33]
Conflicting results have been obtained concerning the importance of testosterone in maintaining cardiovascular health.[34][35] Nevertheless, maintaining normal testosterone levels in elderly men has been shown to improve many parameters that are thought to reduce cardiovascular disease risk, such as increased lean body mass, decreased visceral fat mass, decreased total cholesterol, and glycemic control.[36]
High androgen levels are associated with menstrual cycle irregularities in both clinical populations and healthy women.[37]
Sexual arousal
Testosterone levels follow a nycthemeral rhythm that peaks early each day, regardless of sexual activity.[38]
There are positive correlations between positive orgasm experience in women and testosterone levels where relaxation was a key perception of the experience. There is no correlation between testosterone and men's perceptions of their orgasm experience, and also no correlation between higher testosterone levels and greater sexual assertiveness in either sex.[39]
Sexual arousal and masturbation in women produce small increases in testosterone concentrations.[40] The plasma levels of various steroids significantly increase after masturbation in men and the testosterone levels correlate to those levels.[41]
Mammalian studies
Studies conducted in rats have indicated that their degree of sexual arousal is sensitive to reductions in testosterone. When testosterone-deprived rats were given medium levels of testosterone, their sexual behaviours (copulation, partner preference, etc.) resumed, but not when given low amounts of the same hormone. Therefore, these mammals may provide a model for studying clinical populations among humans suffering from sexual arousal deficits such as hypoactive sexual desire disorder.[42]
Every mammalian species examined demonstrated a marked increase in a male's testosterone level upon encountering a novel female. The reflexive testosterone increases in male mice is related to the male's initial level of sexual arousal.[43]
In non-human primates, it may be that testosterone in puberty stimulates sexual arousal, which allows the primate to increasingly seek out sexual experiences with females and thus creates a sexual preference for females.[44] Some research has also indicated that if testosterone is eliminated in an adult male human or other adult male primate's system, its sexual motivation decreases, but there is no corresponding decrease in ability to engage in sexual activity (mounting, ejaculating, etc.).[44]
In accordance with sperm competition theory, testosterone levels are shown to increase as a response to previously neutral stimuli when conditioned to become sexual in male rats.[45] This reaction engages penile reflexes (such as erection and ejaculation) that aid in sperm competition when more than one male is present in mating encounters, allowing for more production of successful sperm and a higher chance of reproduction.
Males
In men, higher levels of testosterone are associated with periods of sexual activity.[46][47]
Men who watch a sexually explicit movie have an average increase of 35% in testosterone, peaking at 60–90 minutes after the end of the film, but no increase is seen in men who watch sexually neutral films.[48] Men who watch sexually explicit films also report increased motivation, competitiveness, and decreased exhaustion.[49] A link has also been found between relaxation following sexual arousal and testosterone levels.[50]
Men's levels of testosterone, a hormone known to affect men's mating behaviour, changes depending on whether they are exposed to an ovulating or nonovulating woman's body odour. Men who are exposed to scents of ovulating women maintained a stable testosterone level that was higher than the testosterone level of men exposed to nonovulation cues. Men are heavily aware of hormone cycles in females.[51] This may be linked to the ovulatory shift hypothesis,[52] where males are adapted to respond to the ovulation cycles of females by sensing when they are most fertile and whereby females look for preferred male mates when they are the most fertile; both actions may be driven by hormones.
Females
Androgens may modulate the physiology of vaginal tissue and contribute to female genital sexual arousal.[53] Women's level of testosterone is higher when measured pre-intercourse vs pre-cuddling, as well as post-intercourse vs post-cuddling.[54] There is a time lag effect when testosterone is administered, on genital arousal in women. In addition, a continuous increase in vaginal sexual arousal may result in higher genital sensations and sexual appetitive behaviors.[55]
When females have a higher baseline level of testosterone, they have higher increases in sexual arousal levels but smaller increases in testosterone, indicating a ceiling effect on testosterone levels in females. Sexual thoughts also change the level of testosterone but not the level of cortisol in the female body, and hormonal contraceptives may affect the variation in testosterone response to sexual thoughts.[56]
Testosterone may prove to be an effective treatment in female sexual arousal disorders,[57] and is available as a dermal patch. There is no FDA approved androgen preparation for the treatment of androgen insufficiency; however, it has been used as an off-label use to treat low libido and sexual dysfunction in older women. Testosterone may be a treatment for postmenopausal women as long as they are effectively estrogenized.[57]
Romantic relationships
Falling in love decreases men's testosterone levels while increasing women's testosterone levels. There has been speculation that these changes in testosterone result in the temporary reduction of differences in behavior between the sexes.[58] However, it is suggested that after the "honeymoon phase" ends—about four years into a relationship—this change in testosterone levels is no longer apparent.[58] Men who produce less testosterone are more likely to be in a relationship[59] or married,[60] and men who produce more testosterone are more likely to divorce;[60] however, causality cannot be determined in this correlation. Marriage or commitment could cause a decrease in testosterone levels.[61]
Single men who have not had relationship experience have lower testosterone levels than single men with experience. It is suggested that these single men with prior experience are in a more competitive state than their non-experienced counterparts.[62] Married men who engage in bond-maintenance activities such as spending the day with their spouse and/or child have no different testosterone levels compared to times when they do not engage in such activities. Collectively, these results suggest that the presence of competitive activities rather than bond-maintenance activities are more relevant to changes in testosterone levels.[63]
Men who produce more testosterone are more likely to engage in extramarital sex.[60] Testosterone levels do not rely on physical presence of a partner; testosterone levels of men engaging in same-city and long-distance relationships are similar.[59] Physical presence may be required for women who are in relationships for the testosterone–partner interaction, where same-city partnered women have lower testosterone levels than long-distance partnered women.[64]
Fatherhood
Fatherhood decreases testosterone levels in men, suggesting that the emotions and behaviour tied to decreased testosterone promote paternal care. In humans and other species that utilize allomaternal care, paternal investment in offspring is beneficial to said offspring's survival because it allows the parental dyad to raise multiple children simultaneously. This increases the reproductive fitness of the parents because their offspring are more likely to survive and reproduce. Paternal care increases offspring survival due to increased access to higher quality food and reduced physical and immunological threats.[65] This is particularly beneficial for humans since offspring are dependent on parents for extended periods of time and mothers have relatively short inter-birth intervals.[66]
While the extent of paternal care varies between cultures, higher investment in direct child care has been seen to be correlated with lower average testosterone levels as well as temporary fluctuations.[67] For instance, fluctuation in testosterone levels when a child is in distress has been found to be indicative of fathering styles. If a father's testosterone levels decrease in response to hearing their baby cry, it is an indication of empathizing with the baby. This is associated with increased nurturing behavior and better outcomes for the infant.[68]
Aggression and criminality
Most studies support a link between adult criminality and testosterone. Nearly all studies of juvenile delinquency and testosterone are not significant. Most studies have also found testosterone to be associated with behaviors or personality traits linked with criminality such as antisocial behavior and alcoholism. Many studies have also been done on the relationship between more general aggressive behavior and feelings and testosterone. About half the studies have found a relationship and about half no relationship.[71] Studies have also found that testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus.[72]
Testosterone is significantly discussed in relation to aggression and competitive behavior. There are two theories on the role of testosterone in aggression and competition.[73] The first one is the challenge hypothesis which states that testosterone would increase during puberty, thus facilitating reproductive and competitive behavior which would include aggression.[73] It is therefore the challenge of competition among males of the species that facilitates aggression and violence.[73] Studies conducted have found direct correlation between testosterone and dominance, especially among the most violent criminals in prison who had the highest testosterone levels.[73] The same research also found fathers (those outside competitive environments) had the lowest testosterone levels compared to other males.[73]
The second theory is similar and is known as "evolutionary neuroandrogenic (ENA) theory of male aggression".[74][75] Testosterone and other androgens have evolved to masculinize a brain in order to be competitive even to the point of risking harm to the person and others. By doing so, individuals with masculinized brains as a result of pre-natal and adult life testosterone and androgens enhance their resource acquiring abilities in order to survive, attract and copulate with mates as much as possible.[74] The masculinization of the brain is not just mediated by testosterone levels at the adult stage, but also testosterone exposure in the womb as a fetus. Higher pre-natal testosterone indicated by a low digit ratio as well as adult testosterone levels increased risk of fouls or aggression among male players in a soccer game.[76] Studies have also found higher pre-natal testosterone or lower digit ratio to be correlated with higher aggression in males.[77][78][79][80][81]
The rise in testosterone levels during competition predicted aggression in males but not in females.[82] Subjects who interacted with hand guns and an experimental game showed rise in testosterone and aggression.[83] Natural selection might have evolved males to be more sensitive to competitive and status challenge situations and that the interacting roles of testosterone are the essential ingredient for aggressive behaviour in these situations.[84] Testosterone produces aggression by activating subcortical areas in the brain, which may also be inhibited or suppressed by social norms or familial situations while still manifesting in diverse intensities and ways through thoughts, anger, verbal aggression, competition, dominance and physical violence. Testosterone mediates attraction to cruel and violent cues in men by promoting extended viewing of violent stimuli.[85] Testosterone specific structural brain characteristic can predict aggressive behaviour in individuals.[86]
Testosterone might encourage fair behavior. For one study, subjects took part in a behavioral experiment where the distribution of a real amount of money was decided. The rules allowed both fair and unfair offers. The negotiating partner could subsequently accept or decline the offer. The fairer the offer, the less probable a refusal by the negotiating partner. If no agreement was reached, neither party earned anything. Test subjects with an artificially enhanced testosterone level generally made better, fairer offers than those who received placebos, thus reducing the risk of a rejection of their offer to a minimum. Two later studies have empirically confirmed these results.[87][88][89] However men with high testosterone were significantly 27% less generous in an ultimatum game.[90] The Annual NY Academy of Sciences has also found anabolic steroid use (which increases testosterone) to be higher in teenagers, and this was associated with increased violence.[91] Studies have also found administered testosterone to increase verbal aggression and anger in some participants.[92]
A few studies indicate that the testosterone derivative estradiol (one form of estrogen) might play an important role in male aggression.[71][93][94][95] Estradiol is known to correlate with aggression in male mice.[96] Moreover, the conversion of testosterone to estradiol regulates male aggression in sparrows during breeding season.[97] Rats who were given anabolic steroids that increase testosterone were also more physically aggressive to provocation as a result of "threat sensitivity".[98]
The relationship between testosterone and aggression may also function indirectly, as it has been proposed that testosterone does not amplify tendencies towards aggression but rather amplifies whatever tendencies will allow an individual to maintain social status when challenged. In most animals, aggression is the means of maintaining social status. However, humans have multiple ways of obtaining social status. This could explain why some studies find a link between testosterone and pro-social behaviour if pro-social behaviour is rewarded with social status. Thus the link between testosterone and aggression and violence is due to these being rewarded with social status.[99] The relationship may also be one of a "permissive effect" whereby testosterone does elevate aggression levels but only in the sense of allowing average aggression levels to be maintained; chemically or physically castrating the individual will reduce aggression levels (though it will not eliminate them) but the individual only needs a small-level of pre-castration testosterone to have aggression levels to return to normal, which they will remain at even if additional testosterone is added. Testosterone may also simply exaggerate or amplify existing aggression; for example, chimpanzees who receive testosterone increases become more aggressive to chimps lower than them in the social hierarchy but will still be submissive to chimps higher than them. Testosterone thus does not make the chimpanzee indiscriminately aggressive but instead amplifies his pre-existing aggression towards lower-ranked chimps.[100]
In humans, testosterone appears more to promote status-seeking and social dominance than simply increasing physical aggression. When controlling for the effects of belief in having received testosterone, women who have received testosterone make fairer offers than women who have not received testosterone.[101]
Brain
The brain is also affected by this sexual differentiation;[16] the enzyme aromatase converts testosterone into estradiol that is responsible for masculinization of the brain in male mice. In humans, masculinization of the fetal brain appears, by observation of gender preference in patients with congenital diseases of androgen formation or androgen receptor function, to be associated with functional androgen receptors.[102]
There are some differences between a male and female brain (possibly the result of different testosterone levels), one of them being size: the male human brain is, on average, larger.[103] Men were found to have a total myelinated fiber length of 176 000 km at the age of 20, whereas in women the total length was 149 000 km (approx. 15% less).[104]
No immediate short term effects on mood or behavior were found from the administration of supraphysiologic doses of testosterone for 10 weeks on 43 healthy men.[105] A correlation between testosterone and risk tolerance in career choice exists among women.[69][106]
Attention, memory, and spatial ability are key cognitive functions affected by testosterone in humans. Preliminary evidence suggests that low testosterone levels may be a risk factor for cognitive decline and possibly for dementia of the Alzheimer's type,[107][108][109][110] a key argument in life extension medicine for the use of testosterone in anti-aging therapies. Much of the literature, however, suggests a curvilinear or even quadratic relationship between spatial performance and circulating testosterone,[111] where both hypo- and hypersecretion (deficient- and excessive-secretion) of circulating androgens have negative effects on cognition.
Immune system and inflammation
Testosterone deficiency is associated with an increased risk of metabolic syndrome, cardiovascular disease and mortality, which are also sequelae of chronic inflammation.[112] Testosterone plasma concentration inversely correlates to multiple biomarkers of inflammation including CRP, interleukin 1 beta, interleukin 6, TNF alpha and endotoxin concentration, as well as leukocyte count.[112] As demonstrated by a meta-analysis, substitution therapy with testosterone results in a significant reduction of inflammatory markers.[112] These effects are mediated by different mechanisms with synergistic action.[112] In androgen-deficient men with concomitant autoimmune thyroiditis, substitution therapy with testosterone leads to a decrease in thyroid autoantibody titres and an increase in thyroid's secretory capacity (SPINA-GT).[113]
Medical use
Testosterone is used as a medication for the treatment of male hypogonadism, gender dysphoria, and certain types of breast cancer.[12][114] This is known as hormone replacement therapy (HRT) or testosterone replacement therapy (TRT), which maintains serum testosterone levels in the normal range. Decline of testosterone production with age has led to interest in androgen replacement therapy.[115] It is unclear if the use of testosterone for low levels due to aging is beneficial or harmful.[116]
Testosterone is included in the World Health Organization's list of essential medicines, which are the most important medications needed in a basic health system.[117] It is available as a generic medication.[12] It can be administered as a cream or transdermal patch that is applied to the skin, by injection into a muscle, as a tablet that is placed in the cheek, or by ingestion.[12]
Common side effects from testosterone medication include acne, swelling, and breast enlargement in males.[12] Serious side effects may include liver toxicity, heart disease, and behavioral changes.[12] Women and children who are exposed may develop virilization.[12] It is recommended that individuals with prostate cancer not use the medication.[12] It can cause harm if used during pregnancy or breastfeeding.[12]
2020 guidelines from the American College of Physicians support the discussion of testosterone treatment in adult men with age-related low levels of testosterone who have sexual dysfunction. They recommend yearly evaluation regarding possible improvement and, if none, to discontinue testosterone; physicians should consider intramuscular treatments, rather than transdermal treatments, due to costs and since the effectiveness and harm of either method is similar. Testosterone treatment for reasons other than possible improvement of sexual dysfunction may not be recommended.[118][119]
Biological activity
Steroid hormone activity
The effects of testosterone in humans and other vertebrates occur by way of multiple mechanisms: by activation of the androgen receptor (directly or as dihydrotestosterone), and by conversion to estradiol and activation of certain estrogen receptors.[120][121] Androgens such as testosterone have also been found to bind to and activate membrane androgen receptors.[122][123][124]
Free testosterone (T) is transported into the cytoplasm of target tissue cells, where it can bind to the androgen receptor, or can be reduced to 5α-dihydrotestosterone (DHT) by the cytoplasmic enzyme 5α-reductase. DHT binds to the same androgen receptor even more strongly than testosterone, so that its androgenic potency is about 5 times that of T.[125] The T-receptor or DHT-receptor complex undergoes a structural change that allows it to move into the cell nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA. The areas of binding are called hormone response elements (HREs), and influence transcriptional activity of certain genes, producing the androgen effects.
Androgen receptors occur in many different vertebrate body system tissues, and both males and females respond similarly to similar levels. Greatly differing amounts of testosterone prenatally, at puberty, and throughout life account for a share of biological differences between males and females.
The bones and the brain are two important tissues in humans where the primary effect of testosterone is by way of aromatization to estradiol. In the bones, estradiol accelerates ossification of cartilage into bone, leading to closure of the epiphyses and conclusion of growth. In the central nervous system, testosterone is aromatized to estradiol. Estradiol rather than testosterone serves as the most important feedback signal to the hypothalamus (especially affecting LH secretion).[126] In many mammals, prenatal or perinatal "masculinization" of the sexually dimorphic areas of the brain by estradiol derived from testosterone programs later male sexual behavior.[127]
Neurosteroid activity
Testosterone, via its active metabolite 3α-androstanediol, is a potent positive allosteric modulator of the GABAA receptor.[128]
Testosterone has been found to act as an antagonist of the TrkA and p75NTR, receptors for the neurotrophin nerve growth factor (NGF), with high affinity (around 5 nM).[129][130][131] In contrast to testosterone, DHEA and DHEA sulfate have been found to act as high-affinity agonists of these receptors.[129][130][131]
Testosterone is an antagonist of the sigma σ1 receptor (Ki = 1,014 or 201 nM).[132] However, the concentrations of testosterone required for binding the receptor are far above even total circulating concentrations of testosterone in adult males (which range between 10 and 35 nM).[133]
Biochemistry
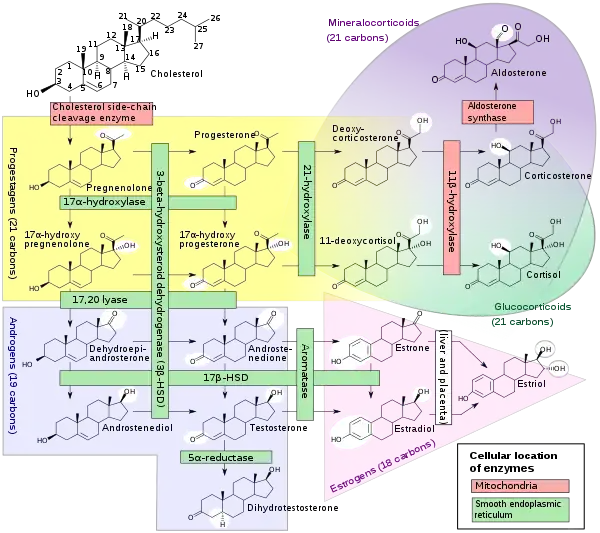
Biosynthesis
Like other steroid hormones, testosterone is derived from cholesterol (see figure).[135] The first step in the biosynthesis involves the oxidative cleavage of the side-chain of cholesterol by cholesterol side-chain cleavage enzyme (P450scc, CYP11A1), a mitochondrial cytochrome P450 oxidase with the loss of six carbon atoms to give pregnenolone. In the next step, two additional carbon atoms are removed by the CYP17A1 (17α-hydroxylase/17,20-lyase) enzyme in the endoplasmic reticulum to yield a variety of C19 steroids.[136] In addition, the 3β-hydroxyl group is oxidized by 3β-hydroxysteroid dehydrogenase to produce androstenedione. In the final and rate limiting step, the C17 keto group androstenedione is reduced by 17β-hydroxysteroid dehydrogenase to yield testosterone.
The largest amounts of testosterone (>95%) are produced by the testes in men,[4] while the adrenal glands account for most of the remainder. Testosterone is also synthesized in far smaller total quantities in women by the adrenal glands, thecal cells of the ovaries, and, during pregnancy, by the placenta.[137] In the testes, testosterone is produced by the Leydig cells.[138] The male generative glands also contain Sertoli cells, which require testosterone for spermatogenesis. Like most hormones, testosterone is supplied to target tissues in the blood where much of it is transported bound to a specific plasma protein, sex hormone-binding globulin (SHBG).
| Sex | Sex hormone | Reproductive phase |
Blood production rate |
Gonadal secretion rate |
Metabolic clearance rate |
Reference range (serum levels) | |
|---|---|---|---|---|---|---|---|
| SI units | Non-SI units | ||||||
| Men | Androstenedione | – |
2.8 mg/day | 1.6 mg/day | 2200 L/day | 2.8–7.3 nmol/L | 80–210 ng/dL |
| Testosterone | – |
6.5 mg/day | 6.2 mg/day | 950 L/day | 6.9–34.7 nmol/L | 200–1000 ng/dL | |
| Estrone | – |
150 μg/day | 110 μg/day | 2050 L/day | 37–250 pmol/L | 10–70 pg/mL | |
| Estradiol | – |
60 μg/day | 50 μg/day | 1600 L/day | <37–210 pmol/L | 10–57 pg/mL | |
| Estrone sulfate | – |
80 μg/day | Insignificant | 167 L/day | 600–2500 pmol/L | 200–900 pg/mL | |
| Women | Androstenedione | – |
3.2 mg/day | 2.8 mg/day | 2000 L/day | 3.1–12.2 nmol/L | 89–350 ng/dL |
| Testosterone | – |
190 μg/day | 60 μg/day | 500 L/day | 0.7–2.8 nmol/L | 20–81 ng/dL | |
| Estrone | Follicular phase | 110 μg/day | 80 μg/day | 2200 L/day | 110–400 pmol/L | 30–110 pg/mL | |
| Luteal phase | 260 μg/day | 150 μg/day | 2200 L/day | 310–660 pmol/L | 80–180 pg/mL | ||
| Postmenopause | 40 μg/day | Insignificant | 1610 L/day | 22–230 pmol/L | 6–60 pg/mL | ||
| Estradiol | Follicular phase | 90 μg/day | 80 μg/day | 1200 L/day | <37–360 pmol/L | 10–98 pg/mL | |
| Luteal phase | 250 μg/day | 240 μg/day | 1200 L/day | 699–1250 pmol/L | 190–341 pg/mL | ||
| Postmenopause | 6 μg/day | Insignificant | 910 L/day | <37–140 pmol/L | 10–38 pg/mL | ||
| Estrone sulfate | Follicular phase | 100 μg/day | Insignificant | 146 L/day | 700–3600 pmol/L | 250–1300 pg/mL | |
| Luteal phase | 180 μg/day | Insignificant | 146 L/day | 1100–7300 pmol/L | 400–2600 pg/mL | ||
| Progesterone | Follicular phase | 2 mg/day | 1.7 mg/day | 2100 L/day | 0.3–3 nmol/L | 0.1–0.9 ng/mL | |
| Luteal phase | 25 mg/day | 24 mg/day | 2100 L/day | 19–45 nmol/L | 6–14 ng/mL | ||
Notes and sources
Notes: "The concentration of a steroid in the circulation is determined by the rate at which it is secreted from glands, the rate of metabolism of precursor or prehormones into the steroid, and the rate at which it is extracted by tissues and metabolized. The secretion rate of a steroid refers to the total secretion of the compound from a gland per unit time. Secretion rates have been assessed by sampling the venous effluent from a gland over time and subtracting out the arterial and peripheral venous hormone concentration. The metabolic clearance rate of a steroid is defined as the volume of blood that has been completely cleared of the hormone per unit time. The production rate of a steroid hormone refers to entry into the blood of the compound from all possible sources, including secretion from glands and conversion of prohormones into the steroid of interest. At steady state, the amount of hormone entering the blood from all sources will be equal to the rate at which it is being cleared (metabolic clearance rate) multiplied by blood concentration (production rate = metabolic clearance rate × concentration). If there is little contribution of prohormone metabolism to the circulating pool of steroid, then the production rate will approximate the secretion rate." Sources: See template. | |||||||
Regulation
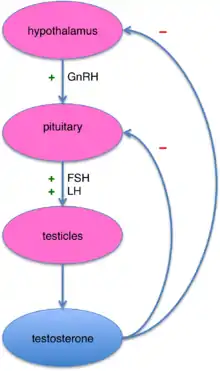
In males, testosterone is synthesized primarily in Leydig cells. The number of Leydig cells in turn is regulated by luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In addition, the amount of testosterone produced by existing Leydig cells is under the control of LH, which regulates the expression of 17β-hydroxysteroid dehydrogenase.[139]
The amount of testosterone synthesized is regulated by the hypothalamic–pituitary–testicular axis (see figure to the right).[140] When testosterone levels are low, gonadotropin-releasing hormone (GnRH) is released by the hypothalamus, which in turn stimulates the pituitary gland to release FSH and LH. These latter two hormones stimulate the testis to synthesize testosterone. Finally, increasing levels of testosterone through a negative feedback loop act on the hypothalamus and pituitary to inhibit the release of GnRH and FSH/LH, respectively.
Factors affecting testosterone levels may include:
- Age: Testosterone levels gradually reduce as men age.[141][142] This effect is sometimes referred to as andropause or late-onset hypogonadism.[143]
- Exercise: Resistance training increases testosterone levels,[144] however, in older men, that increase can be avoided by protein ingestion.[145] Endurance training in men may lead to lower testosterone levels.[146]
- Nutrients: Vitamin A deficiency may lead to sub-optimal plasma testosterone levels.[147] The secosteroid vitamin D in levels of 400–1000 IU/d (10–25 µg/d) raises testosterone levels.[148] Zinc deficiency lowers testosterone levels[149] but over-supplementation has no effect on serum testosterone.[150]
- Weight loss: Reduction in weight may result in an increase in testosterone levels. Fat cells synthesize the enzyme aromatase, which converts testosterone, the male sex hormone, into estradiol, the female sex hormone.[151] However no clear association between body mass index and testosterone levels has been found.[152]
- Miscellaneous: Sleep: (REM sleep) increases nocturnal testosterone levels.[153] Behavior: Dominance challenges can, in some cases, stimulate increased testosterone release in men.[154] Drugs: Natural or man-made antiandrogens including spearmint tea reduce testosterone levels.[155][156][157] Licorice can decrease the production of testosterone and this effect is greater in females.[158]
Distribution
The plasma protein binding of testosterone is 98.0 to 98.5%, with 1.5 to 2.0% free or unbound.[159] It is bound 65% to sex hormone-binding globulin (SHBG) and 33% bound weakly to albumin.[160]
| Compound | Group | Level (nM) | Free (%) | SHBG (%) | CBG (%) | Albumin (%) |
|---|---|---|---|---|---|---|
| Testosterone | Adult men | 23.0 | 2.23 | 44.3 | 3.56 | 49.9 |
| Adult women | ||||||
| Follicular phase | 1.3 | 1.36 | 66.0 | 2.26 | 30.4 | |
| Luteal phase | 1.3 | 1.37 | 65.7 | 2.20 | 30.7 | |
| Pregnancy | 4.7 | 0.23 | 95.4 | 0.82 | 3.6 | |
| Dihydrotestosterone | Adult men | 1.70 | 0.88 | 49.7 | 0.22 | 39.2 |
| Adult women | ||||||
| Follicular phase | 0.65 | 0.47 | 78.4 | 0.12 | 21.0 | |
| Luteal phase | 0.65 | 0.48 | 78.1 | 0.12 | 21.3 | |
| Pregnancy | 0.93 | 0.07 | 97.8 | 0.04 | 21.2 | |
| Sources: See template. | ||||||
Metabolism
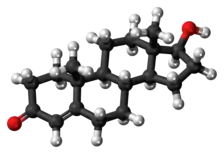
Testosterone metabolism in humans
|
Both testosterone and 5α-DHT are metabolized mainly in the liver.[2][161] Approximately 50% of testosterone is metabolized via conjugation into testosterone glucuronide and to a lesser extent testosterone sulfate by glucuronosyltransferases and sulfotransferases, respectively.[2] An additional 40% of testosterone is metabolized in equal proportions into the 17-ketosteroids androsterone and etiocholanolone via the combined actions of 5α- and 5β-reductases, 3α-hydroxysteroid dehydrogenase, and 17β-HSD, in that order.[2][161][162] Androsterone and etiocholanolone are then glucuronidated and to a lesser extent sulfated similarly to testosterone.[2][161] The conjugates of testosterone and its hepatic metabolites are released from the liver into circulation and excreted in the urine and bile.[2][161][162] Only a small fraction (2%) of testosterone is excreted unchanged in the urine.[161]
In the hepatic 17-ketosteroid pathway of testosterone metabolism, testosterone is converted in the liver by 5α-reductase and 5β-reductase into 5α-DHT and the inactive 5β-DHT, respectively.[2][161] Then, 5α-DHT and 5β-DHT are converted by 3α-HSD into 3α-androstanediol and 3α-etiocholanediol, respectively.[2][161] Subsequently, 3α-androstanediol and 3α-etiocholanediol are converted by 17β-HSD into androsterone and etiocholanolone, which is followed by their conjugation and excretion.[2][161] 3β-Androstanediol and 3β-etiocholanediol can also be formed in this pathway when 5α-DHT and 5β-DHT are acted upon by 3β-HSD instead of 3α-HSD, respectively, and they can then be transformed into epiandrosterone and epietiocholanolone, respectively.[163][164] A small portion of approximately 3% of testosterone is reversibly converted in the liver into androstenedione by 17β-HSD.[162]
In addition to conjugation and the 17-ketosteroid pathway, testosterone can also be hydroxylated and oxidized in the liver by cytochrome P450 enzymes, including CYP3A4, CYP3A5, CYP2C9, CYP2C19, and CYP2D6.[165] 6β-Hydroxylation and to a lesser extent 16β-hydroxylation are the major transformations.[165] The 6β-hydroxylation of testosterone is catalyzed mainly by CYP3A4 and to a lesser extent CYP3A5 and is responsible for 75 to 80% of cytochrome P450-mediated testosterone metabolism.[165] In addition to 6β- and 16β-hydroxytestosterone, 1β-, 2α/β-, 11β-, and 15β-hydroxytestosterone are also formed as minor metabolites.[165][166] Certain cytochrome P450 enzymes such as CYP2C9 and CYP2C19 can also oxidize testosterone at the C17 position to form androstenedione.[165]
Two of the immediate metabolites of testosterone, 5α-DHT and estradiol, are biologically important and can be formed both in the liver and in extrahepatic tissues.[161] Approximately 5 to 7% of testosterone is converted by 5α-reductase into 5α-DHT, with circulating levels of 5α-DHT about 10% of those of testosterone, and approximately 0.3% of testosterone is converted into estradiol by aromatase.[4][161][167][168] 5α-Reductase is highly expressed in the male reproductive organs (including the prostate gland, seminal vesicles, and epididymides),[169] skin, hair follicles, and brain[170] and aromatase is highly expressed in adipose tissue, bone, and the brain.[171][172] As much as 90% of testosterone is converted into 5α-DHT in so-called androgenic tissues with high 5α-reductase expression,[162] and due to the several-fold greater potency of 5α-DHT as an AR agonist relative to testosterone,[173] it has been estimated that the effects of testosterone are potentiated 2- to 3-fold in such tissues.[174]
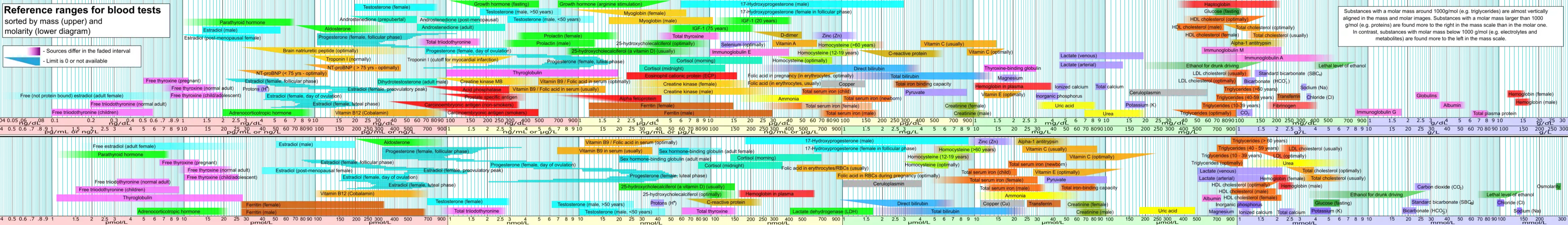
Levels
Total levels of testosterone in the body are 264 to 916 ng/dL in men age 19 to 39 years,[175] while mean testosterone levels in adult men have been reported as 630 ng/dL.[176] Levels of testosterone in men decline with age.[175] In women, mean levels of total testosterone have been reported to be 32.6 ng/dL.[177][178] In women with hyperandrogenism, mean levels of total testosterone have been reported to be 62.1 ng/dL.[177][178]
| Total testosterone | |||||
|---|---|---|---|---|---|
| Stage | Age range | Male | Female | ||
| Values | SI units | Values | SI units | ||
| Infant | Premature (26–28 weeks) | 59–125 ng/dL | 2.047–4.337 nmol/L | 5–16 ng/dL | 0.173–0.555 nmol/L |
| Premature (31–35 weeks) | 37–198 ng/dL | 1.284–6.871 nmol/L | 5–22 ng/dL | 0.173–0.763 nmol/L | |
| Newborn | 75–400 ng/dL | 2.602–13.877 nmol/L | 20–64 ng/dL | 0.694–2.220 nmol/L | |
| Child | 1–6 years | ND | ND | ND | ND |
| 7–9 years | 0–8 ng/dL | 0–0.277 nmol/L | 1–12 ng/dL | 0.035–0.416 nmol/L | |
| Just before puberty | 3–10 ng/dL* | 0.104–0.347 nmol/L* | <10 ng/dL* | <0.347 nmol/L* | |
| Puberty | 10–11 years | 1–48 ng/dL | 0.035–1.666 nmol/L | 2–35 ng/dL | 0.069–1.214 nmol/L |
| 12–13 years | 5–619 ng/dL | 0.173–21.480 nmol/L | 5–53 ng/dL | 0.173–1.839 nmol/L | |
| 14–15 years | 100–320 ng/dL | 3.47–11.10 nmol/L | 8–41 ng/dL | 0.278–1.423 nmol/L | |
| 16–17 years | 200–970 ng/dL* | 6.94–33.66 nmol/L* | 8–53 ng/dL | 0.278–1.839 nmol/L | |
| Adult | ≥18 years | 350–1080 ng/dL* | 12.15–37.48 nmol/L* | – | – |
| 20–39 years | 400–1080 ng/dL | 13.88–37.48 nmol/L | – | – | |
| 40–59 years | 350–890 ng/dL | 12.15–30.88 nmol/L | – | – | |
| ≥60 years | 350–720 ng/dL | 12.15–24.98 nmol/L | – | – | |
| Premenopausal | – | – | 10–54 ng/dL | 0.347–1.873 nmol/L | |
| Postmenopausal | – | – | 7–40 ng/dL | 0.243–1.388 nmol/L | |
| Bioavailable testosterone | |||||
| Stage | Age range | Male | Female | ||
| Values | SI units | Values | SI units | ||
| Child | 1–6 years | 0.2–1.3 ng/dL | 0.007–0.045 nmol/L | 0.2–1.3 ng/dL | 0.007–0.045 nmol/L |
| 7–9 years | 0.2–2.3 ng/dL | 0.007–0.079 nmol/L | 0.2–4.2 ng/dL | 0.007–0.146 nmol/L | |
| Puberty | 10–11 years | 0.2–14.8 ng/dL | 0.007–0.513 nmol/L | 0.4–19.3 ng/dL | 0.014–0.670 nmol/L |
| 12–13 years | 0.3–232.8 ng/dL | 0.010–8.082 nmol/L | 1.1–15.6 ng/dL | 0.038–0.541 nmol/L | |
| 14–15 years | 7.9–274.5 ng/dL | 0.274–9.525 nmol/L | 2.5–18.8 ng/dL | 0.087–0.652 nmol/L | |
| 16–17 years | 24.1–416.5 ng/dL | 0.836–14.452 nmol/L | 2.7–23.8 ng/dL | 0.094–0.826 nmol/L | |
| Adult | ≥18 years | ND | ND | – | – |
| Premenopausal | – | – | 1.9–22.8 ng/dL | 0.066–0.791 nmol/L | |
| Postmenopausal | – | – | 1.6–19.1 ng/dL | 0.055–0.662 nmol/L | |
| Free testosterone | |||||
| Stage | Age range | Male | Female | ||
| Values | SI units | Values | SI units | ||
| Child | 1–6 years | 0.1–0.6 pg/mL | 0.3–2.1 pmol/L | 0.1–0.6 pg/mL | 0.3–2.1 pmol/L |
| 7–9 years | 0.1–0.8 pg/mL | 0.3–2.8 pmol/L | 0.1–1.6 pg/mL | 0.3–5.6 pmol/L | |
| Puberty | 10–11 years | 0.1–5.2 pg/mL | 0.3–18.0 pmol/L | 0.1–2.9 pg/mL | 0.3–10.1 pmol/L |
| 12–13 years | 0.4–79.6 pg/mL | 1.4–276.2 pmol/L | 0.6–5.6 pg/mL | 2.1–19.4 pmol/L | |
| 14–15 years | 2.7–112.3 pg/mL | 9.4–389.7 pmol/L | 1.0–6.2 pg/mL | 3.5–21.5 pmol/L | |
| 16–17 years | 31.5–159 pg/mL | 109.3–551.7 pmol/L | 1.0–8.3 pg/mL | 3.5–28.8 pmol/L | |
| Adult | ≥18 years | 44–244 pg/mL | 153–847 pmol/L | – | – |
| Premenopausal | – | – | 0.8–9.2 pg/mL | 2.8–31.9 pmol/L | |
| Postmenopausal | – | – | 0.6–6.7 pg/mL | 2.1–23.2 pmol/L | |
| Sources: See template. | |||||
| Life stage | Tanner stage | Age range | Mean age | Levels range | Mean levels |
|---|---|---|---|---|---|
| Child | Stage I | <10 years | – | <30 ng/dL | 5.8 ng/dL |
| Puberty | Stage II | 10–14 years | 12 years | <167 ng/dL | 40 ng/dL |
| Stage III | 12–16 years | 13–14 years | 21–719 ng/dL | 190 ng/dL | |
| Stage IV | 13–17 years | 14–15 years | 25–912 ng/dL | 370 ng/dL | |
| Stage V | 13–17 years | 15 years | 110–975 ng/dL | 550 ng/dL | |
| Adult | – | ≥18 years | – | 250–1,100 ng/dL | 630 ng/dL |
| Sources: [179][180][176][181][182] | |||||
Measurement
Testosterone's bioavailable concentration is commonly determined using the Vermeulen calculation or more precisely using the modified Vermeulen method,[183][184] which considers the dimeric form of sex-hormone-binding-globulin.[185]
Both methods use chemical equilibrium to derive the concentration of bioavailable testosterone: in circulation, testosterone has two major binding partners, albumin (weakly bound) and sex-hormone-binding-globulin (strongly bound). These methods are described in detail in the accompanying figure.
 Dimeric sex-hormone-binding-globulin with its testosterone ligands
Dimeric sex-hormone-binding-globulin with its testosterone ligands Two methods for determining the concentration of bioavailable testosterone.
Two methods for determining the concentration of bioavailable testosterone.
History

A testicular action was linked to circulating blood fractions – now understood to be a family of androgenic hormones – in the early work on castration and testicular transplantation in fowl by Arnold Adolph Berthold (1803–1861).[186] Research on the action of testosterone received a brief boost in 1889, when the Harvard professor Charles-Édouard Brown-Séquard (1817–1894), then in Paris, self-injected subcutaneously a "rejuvenating elixir" consisting of an extract of dog and guinea pig testicle. He reported in The Lancet that his vigor and feeling of well-being were markedly restored but the effects were transient,[187] and Brown-Séquard's hopes for the compound were dashed. Suffering the ridicule of his colleagues, he abandoned his work on the mechanisms and effects of androgens in human beings.
In 1927, the University of Chicago's Professor of Physiologic Chemistry, Fred C. Koch, established easy access to a large source of bovine testicles — the Chicago stockyards — and recruited students willing to endure the tedious work of extracting their isolates. In that year, Koch and his student, Lemuel McGee, derived 20 mg of a substance from a supply of 40 pounds of bovine testicles that, when administered to castrated roosters, pigs and rats, re-masculinized them.[188] The group of Ernst Laqueur at the University of Amsterdam purified testosterone from bovine testicles in a similar manner in 1934, but the isolation of the hormone from animal tissues in amounts permitting serious study in humans was not feasible until three European pharmaceutical giants—Schering (Berlin, Germany), Organon (Oss, Netherlands) and Ciba (Basel, Switzerland)—began full-scale steroid research and development programs in the 1930s.
The Organon group in the Netherlands were the first to isolate the hormone, identified in a May 1935 paper "On Crystalline Male Hormone from Testicles (Testosterone)".[189] They named the hormone testosterone, from the stems of testicle and sterol, and the suffix of ketone. The structure was worked out by Schering's Adolf Butenandt, at the Chemisches Institut of Technical University in Gdańsk.[190][191]
The chemical synthesis of testosterone from cholesterol was achieved in August that year by Butenandt and Hanisch.[192] Only a week later, the Ciba group in Zurich, Leopold Ruzicka (1887–1976) and A. Wettstein, published their synthesis of testosterone.[193] These independent partial syntheses of testosterone from a cholesterol base earned both Butenandt and Ruzicka the joint 1939 Nobel Prize in Chemistry.[191][194] Testosterone was identified as 17β-hydroxyandrost-4-en-3-one (C19H28O2), a solid polycyclic alcohol with a hydroxyl group at the 17th carbon atom. This also made it obvious that additional modifications on the synthesized testosterone could be made, i.e., esterification and alkylation.
The partial synthesis in the 1930s of abundant, potent testosterone esters permitted the characterization of the hormone's effects, so that Kochakian and Murlin (1936) were able to show that testosterone raised nitrogen retention (a mechanism central to anabolism) in the dog, after which Allan Kenyon's group[195] was able to demonstrate both anabolic and androgenic effects of testosterone propionate in eunuchoidal men, boys, and women. The period of the early 1930s to the 1950s has been called "The Golden Age of Steroid Chemistry",[196] and work during this period progressed quickly.[197]
Other species
Testosterone is observed in most vertebrates. Testosterone and the classical nuclear androgen receptor first appeared in gnathostomes (jawed vertebrates).[198] Agnathans (jawless vertebrates) such as lampreys do not produce testosterone but instead use androstenedione as a male sex hormone.[199] Fish make a slightly different form called 11-ketotestosterone.[200] Its counterpart in insects is ecdysone.[201] The presence of these ubiquitous steroids in a wide range of animals suggest that sex hormones have an ancient evolutionary history.[202]
References
- Haynes WM, ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 3.304. ISBN 978-1439855119.
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (November 30, 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 711–. ISBN 978-0-323-29738-7.
- "Understanding the risks of performance-enhancing drugs". Mayo Clinic. Retrieved December 30, 2019.
- Mooradian AD, Morley JE, Korenman SG (February 1987). "Biological actions of androgens". Endocrine Reviews. 8 (1): 1–28. doi:10.1210/edrv-8-1-1. PMID 3549275.
- Bassil N, Alkaade S, Morley JE (June 2009). "The benefits and risks of testosterone replacement therapy: a review". Therapeutics and Clinical Risk Management. 5 (3): 427–48. doi:10.2147/tcrm.s3025. PMC 2701485. PMID 19707253.
- Tuck SP, Francis RM (2009). "Testosterone, bone and osteoporosis". Advances in the Management of Testosterone Deficiency. Frontiers of Hormone Research. 37. pp. 123–32. doi:10.1159/000176049. ISBN 978-3-8055-8622-1. PMID 19011293.
- Luetjens CM, Weinbauer GF (2012). "Chapter 2: Testosterone: Biosynthesis, transport, metabolism and (non-genomic) actions". In Nieschlag E, Behre HM, Nieschlag S (eds.). Testosterone: Action, Deficiency, Substitution (4th ed.). Cambridge: Cambridge University Press. pp. 15–32. ISBN 978-1-107-01290-5.
- Torjesen PA, Sandnes L (March 2004). "Serum testosterone in women as measured by an automated immunoassay and a RIA". Clinical Chemistry. 50 (3): 678, author reply 678–9. doi:10.1373/clinchem.2003.027565. PMID 14981046.
- Southren AL, Gordon GG, Tochimoto S, Pinzon G, Lane DR, Stypulkowski W (May 1967). "Mean plasma concentration, metabolic clearance and basal plasma production rates of testosterone in normal young men and women using a constant infusion procedure: effect of time of day and plasma concentration on the metabolic clearance rate of testosterone". The Journal of Clinical Endocrinology & Metabolism. 27 (5): 686–94. doi:10.1210/jcem-27-5-686. PMID 6025472.
- Southren AL, Tochimoto S, Carmody NC, Isurugi K (November 1965). "Plasma production rates of testosterone in normal adult men and women and in patients with the syndrome of feminizing testes". The Journal of Clinical Endocrinology & Metabolism. 25 (11): 1441–50. doi:10.1210/jcem-25-11-1441. PMID 5843701.
- Dabbs M, Dabbs JM (2000). Heroes, rogues, and lovers: testosterone and behavior. New York: McGraw-Hill. ISBN 978-0-07-135739-5.
- "Testosterone". Drugs.com. American Society of Health-System Pharmacists. December 4, 2015. Archived from the original on August 20, 2016. Retrieved September 3, 2016.
- Institute of Medicine (US) Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy, Liverman CT, Blazer DG (2004). "Introduction". Testosterone and Aging: Clinical Research Directions (Report). National Academies Press (US).
- Sheffield-Moore M (2000). "Androgens and the control of skeletal muscle protein synthesis". Annals of Medicine. 32 (3): 181–6. doi:10.3109/07853890008998825. PMID 10821325. S2CID 32366484.
- Handelsman DJ (January 2013). "Androgen Physiology, Pharmacology and Abuse". Endotext [Internet]. WWW.ENDOTEXT.ORG. MDText.com, Inc.
- Swaab DF, Garcia-Falgueras A (2009). "Sexual differentiation of the human brain in relation to gender identity and sexual orientation". Functional Neurology. 24 (1): 17–28. PMID 19403051.
- Xu HY, Zhang HX, Xiao Z, Qiao J, Li R (2019). "Regulation of anti-Müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility". Asian Journal of Andrology. 21 (2): 109–114. doi:10.4103/aja.aja_83_18. PMC 6413543. PMID 30381580.
- Berenbaum SA (March 2018). "Beyond Pink and Blue: The Complexity of Early Androgen Effects on Gender Development". Child Development Perspectives. 12 (1): 58–64. doi:10.1111/cdep.12261. PMC 5935256. PMID 29736184.
- Hines M, Brook C, Conway GS (February 2004). "Androgen and psychosexual development: core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH)". Journal of Sex Research. 41 (1): 75–81. doi:10.1080/00224490409552215. PMID 15216426. S2CID 33519930.
- Forest MG, Cathiard AM, Bertrand JA (July 1973). "Evidence of testicular activity in early infancy". The Journal of Clinical Endocrinology & Metabolism. 37 (1): 148–51. doi:10.1210/jcem-37-1-148. PMID 4715291.
- Corbier P, Edwards DA, Roffi J (1992). "The neonatal testosterone surge: a comparative study". Archives Internationales de Physiologie, de Biochimie et de Biophysique. 100 (2): 127–31. doi:10.3109/13813459209035274. PMID 1379488.
- Dakin CL, Wilson CA, Kalló I, Coen CW, Davies DC (May 2008). "Neonatal stimulation of 5-HT(2) receptors reduces androgen receptor expression in the rat anteroventral periventricular nucleus and sexually dimorphic preoptic area". The European Journal of Neuroscience. 27 (9): 2473–80. doi:10.1111/j.1460-9568.2008.06216.x. PMID 18445234. S2CID 23978105.
- Kalat JW (2009). "Reproductive behaviors". Biological psychology. Belmont, Calif: Wadsworth, Cengage Learning. p. 321. ISBN 978-0-495-60300-9.
- Pinyerd B, Zipf WB (2005). "Puberty-timing is everything!". Journal of Pediatric Nursing. 20 (2): 75–82. doi:10.1016/j.pedn.2004.12.011. PMID 15815567.
- Ganong (2012). Ganong's Review of Medical Physiology (24 ed.). TATA McGRAW Hill. pp. 423–25. ISBN 978-1-25-902753-6.
- Raggatt LJ, Partridge NC (2010). "Cellular and molecular mechanisms of bone remodeling". The Journal of Biological Chemistry. 285 (33): 25103–8. doi:10.1074/jbc.R109.041087. PMC 2919071. PMID 20501658.
- Mehta PH, Jones AC, Josephs RA (June 2008). "The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat" (PDF). Journal of Personality and Social Psychology. 94 (6): 1078–93. CiteSeerX 10.1.1.336.2502. doi:10.1037/0022-3514.94.6.1078. PMID 18505319. Archived from the original (PDF) on April 19, 2009.
- Ajayi AA, Halushka PV (May 2005). "Castration reduces platelet thromboxane A2 receptor density and aggregability". QJM. 98 (5): 349–56. doi:10.1093/qjmed/hci054. PMID 15820970.
- Ajayi AA, Mathur R, Halushka PV (June 1995). "Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses". Circulation. 91 (11): 2742–7. doi:10.1161/01.CIR.91.11.2742. PMID 7758179.
- Kelsey TW, Li LQ, Mitchell RT, Whelan A, Anderson RA, Wallace WH (October 8, 2014). "A validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years". PLOS ONE. 9 (10): e109346. Bibcode:2014PLoSO...9j9346K. doi:10.1371/journal.pone.0109346. PMC 4190174. PMID 25295520.
- Morgentaler A, Schulman C (2009). "Testosterone and prostate safety". Advances in the Management of Testosterone Deficiency. Frontiers of Hormone Research. 37. pp. 197–203. doi:10.1159/000176054. ISBN 978-3-8055-8622-1. PMID 19011298.
- Rhoden EL, Averbeck MA, Teloken PE (September 2008). "Androgen replacement in men undergoing treatment for prostate cancer". The Journal of Sexual Medicine. 5 (9): 2202–08. doi:10.1111/j.1743-6109.2008.00925.x. PMID 18638000.
- Morgentaler A, Traish AM (February 2009). "Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth". European Urology. 55 (2): 310–20. doi:10.1016/j.eururo.2008.09.024. PMID 18838208.
- Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, Uraga MV, Erwin PJ, Montori VM (January 2007). "Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials". Mayo Clinic Proceedings. 82 (1): 29–39. doi:10.4065/82.1.29. PMID 17285783.
- Jones TH, Saad F (December 2009). "The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process". Atherosclerosis. 207 (2): 318–27. doi:10.1016/j.atherosclerosis.2009.04.016. PMID 19464009.
- Stanworth RD, Jones TH (2008). "Testosterone for the aging male; current evidence and recommended practice". Clinical Interventions in Aging. 3 (1): 25–44. doi:10.2147/CIA.S190. PMC 2544367. PMID 18488876.
- Van Anders SM, Watson NV (2006). "Menstrual cycle irregularities are associated with testosterone levels in healthy premenopausal women" (PDF). American Journal of Human Biology. 18 (6): 841–44. doi:10.1002/ajhb.20555. hdl:2027.42/83925. PMID 17039468. S2CID 32023452.
- Fox CA, Ismail AA, Love DN, Kirkham KE, Loraine JA (January 1972). "Studies on the relationship between plasma testosterone levels and human sexual activity". The Journal of Endocrinology. 52 (1): 51–8. doi:10.1677/joe.0.0520051. PMID 5061159.
- van Anders SM, Dunn EJ (August 2009). "Are gonadal steroids linked with orgasm perceptions and sexual assertiveness in women and men?". Hormones and Behavior. 56 (2): 206–13. doi:10.1016/j.yhbeh.2009.04.007. hdl:2027.42/83876. PMID 19409392. S2CID 14588630.
- Exton MS, Bindert A, Krüger T, Scheller F, Hartmann U, Schedlowski M (1999). "Cardiovascular and endocrine alterations after masturbation-induced orgasm in women". Psychosomatic Medicine. 61 (3): 280–89. doi:10.1097/00006842-199905000-00005. PMID 10367606.
- Purvis K, Landgren BM, Cekan Z, Diczfalusy E (September 1976). "Endocrine effects of masturbation in men". The Journal of Endocrinology. 70 (3): 439–44. doi:10.1677/joe.0.0700439. PMID 135817.
- Harding SM, Velotta JP (May 2011). "Comparing the relative amount of testosterone required to restore sexual arousal, motivation, and performance in male rats". Hormones and Behavior. 59 (5): 666–73. doi:10.1016/j.yhbeh.2010.09.009. PMID 20920505. S2CID 1577450.
- James PJ, Nyby JG, Saviolakis GA (September 2006). "Sexually stimulated testosterone release in male mice (Mus musculus): roles of genotype and sexual arousal". Hormones and Behavior. 50 (3): 424–31. doi:10.1016/j.yhbeh.2006.05.004. PMID 16828762. S2CID 36436418.
- Wallen K (September 2001). "Sex and context: hormones and primate sexual motivation". Hormones and Behavior. 40 (2): 339–57. CiteSeerX 10.1.1.22.5968. doi:10.1006/hbeh.2001.1696. PMID 11534996. S2CID 2214664.
- Hart BL (December 1983). "Role of testosterone secretion and penile reflexes in sexual behavior and sperm competition in male rats: a theoretical contribution". Physiology & Behavior. 31 (6): 823–27. doi:10.1016/0031-9384(83)90279-2. PMID 6665072. S2CID 42155431.
- Kraemer HC, Becker HB, Brodie HK, Doering CH, Moos RH, Hamburg DA (March 1976). "Orgasmic frequency and plasma testosterone levels in normal human males". Archives of Sexual Behavior. 5 (2): 125–32. doi:10.1007/BF01541869. PMID 1275688. S2CID 38283107.
- Roney JR, Mahler SV, Maestripieri D (2003). "Behavioral and hormonal responses of men to brief interactions with women". Evolution and Human Behavior. 24 (6): 365–75. doi:10.1016/S1090-5138(03)00053-9.
- Pirke KM, Kockott G, Dittmar F (November 1974). "Psychosexual stimulation and plasma testosterone in man". Archives of Sexual Behavior. 3 (6): 577–84. doi:10.1007/BF01541140. PMID 4429441. S2CID 43495791.
- Hellhammer DH, Hubert W, Schürmeyer T (1985). "Changes in saliva testosterone after psychological stimulation in men". Psychoneuroendocrinology. 10 (1): 77–81. doi:10.1016/0306-4530(85)90041-1. PMID 4001279. S2CID 41819670.
- Rowland DL, Heiman JR, Gladue BA, Hatch JP, Doering CH, Weiler SJ (1987). "Endocrine, psychological and genital response to sexual arousal in men". Psychoneuroendocrinology. 12 (2): 149–58. doi:10.1016/0306-4530(87)90045-X. PMID 3602262. S2CID 35309934.
- Miller SL, Maner JK (February 2010). "Scent of a woman: men's testosterone responses to olfactory ovulation cues". Psychological Science. 21 (2): 276–83. doi:10.1177/0956797609357733. PMID 20424057. S2CID 18170407.
- Gangestead SW, Thornhill R, Garver-Apgar CE (2005). "Adaptations to Ovulation: Implications for Sexual and Social Behavior". Current Directions in Psychological Science. 14 (6): 312–16. doi:10.1111/j.0963-7214.2005.00388.x. S2CID 53074076.
- Traish AM, Kim N, Min K, Munarriz R, Goldstein I (April 2002). "Role of androgens in female genital sexual arousal: receptor expression, structure, and function". Fertility and Sterility. 77 Suppl 4: S11–8. doi:10.1016/s0015-0282(02)02978-3. PMID 12007897.
- van Anders SM, Hamilton LD, Schmidt N, Watson NV (April 2007). "Associations between testosterone secretion and sexual activity in women". Hormones and Behavior. 51 (4): 477–82. doi:10.1016/j.yhbeh.2007.01.003. hdl:2027.42/83880. PMID 17320881. S2CID 5718960.
- Tuiten A, Van Honk J, Koppeschaar H, Bernaards C, Thijssen J, Verbaten R (February 2000). "Time course of effects of testosterone administration on sexual arousal in women". Archives of General Psychiatry. 57 (2): 149–53, discussion 155–6. doi:10.1001/archpsyc.57.2.149. PMID 10665617.
- Goldey KL, van Anders SM (May 2011). "Sexy thoughts: effects of sexual cognitions on testosterone, cortisol, and arousal in women" (PDF). Hormones and Behavior. 59 (5): 754–64. doi:10.1016/j.yhbeh.2010.12.005. hdl:2027.42/83874. PMID 21185838. S2CID 18691358.
- Bolour S, Braunstein G (2005). "Testosterone therapy in women: a review". International Journal of Impotence Research. 17 (5): 399–408. doi:10.1038/sj.ijir.3901334. PMID 15889125.
- Marazziti D, Canale D (August 2004). "Hormonal changes when falling in love". Psychoneuroendocrinology. 29 (7): 931–36. doi:10.1016/j.psyneuen.2003.08.006. PMID 15177709. S2CID 24651931.
- van Anders SM, Watson NV (July 2006). "Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: cross-sectional and longitudinal data". Psychoneuroendocrinology. 31 (6): 715–23. doi:10.1016/j.psyneuen.2006.01.008. hdl:2027.42/83924. PMID 16621328. S2CID 22477678.
- Booth A, Dabbs JM (1993). "Testosterone and Men's Marriages". Social Forces. 72 (2): 463–77. doi:10.1093/sf/72.2.463.
- Mazur A, Michalek J (1998). "Marriage, Divorce, and Male Testosterone". Social Forces. 77 (1): 315–30. doi:10.1093/sf/77.1.315.
- Gray PB, Chapman JF, Burnham TC, McIntyre MH, Lipson SF, Ellison PT (June 2004). "Human male pair bonding and testosterone". Human Nature. 15 (2): 119–31. doi:10.1007/s12110-004-1016-6. PMID 26190409. S2CID 33812118.
- Gray PB, Campbell BC, Marlowe FW, Lipson SF, Ellison PT (October 2004). "Social variables predict between-subject but not day-to-day variation in the testosterone of US men". Psychoneuroendocrinology. 29 (9): 1153–62. doi:10.1016/j.psyneuen.2004.01.008. PMID 15219639. S2CID 18107730.
- van Anders SM, Watson NV (February 2007). "Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships". Hormones and Behavior. 51 (2): 286–91. doi:10.1016/j.yhbeh.2006.11.005. PMID 17196592. S2CID 30710035.
- Bribiescas RG, Ellison PT, Gray PB (December 2012). "Male Life History, Reproductive Effort, and the Evolution of the Genus Homo". Current Anthropology. 53 (S6): S424–S435. doi:10.1086/667538. S2CID 83046141.
- Kramer KL, Otárola-Castillo E (July 2015). "When mothers need others: The impact of hominin life history evolution on cooperative breeding". Journal of Human Evolution. 84: 16–24. doi:10.1016/j.jhevol.2015.01.009. PMID 25843884.
- Gettler LT (July 8, 2014). "Applying socioendocrinology to evolutionary models: fatherhood and physiology". Evolutionary Anthropology. 23 (4): 146–60. doi:10.1002/evan.21412. PMID 25116846. S2CID 438574.
- Nauert R (October 30, 2015). "Parenting Skills Influenced by Testosterone Levels, Empathy". Psych Central.
- Sapienza P, Zingales L, Maestripieri D (September 2009). "Gender differences in financial risk aversion and career choices are affected by testosterone". Proceedings of the National Academy of Sciences of the United States of America. 106 (36): 15268–73. Bibcode:2009PNAS..10615268S. doi:10.1073/pnas.0907352106. PMC 2741240. PMID 19706398.
- Apicella CL, Dreber A, Campbell B, Gray PB, Hoffman M, Little AC (November 2008). "Testosterone and financial risk preferences". Evolution and Human Behavior. 29 (6): 384–90. doi:10.1016/j.evolhumbehav.2008.07.001.
- Wright J, Ellis L, Beaver K (2009). Handbook of crime correlates. San Diego: Academic Press. pp. 208–10. ISBN 978-0-12-373612-3.
- Delville Y, Mansour KM, Ferris CF (July 1996). "Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus". Physiology & Behavior. 60 (1): 25–9. doi:10.1016/0031-9384(95)02246-5. PMID 8804638. S2CID 23870320.
- Archer J (2006). "Testosterone and human aggression: an evaluation of the challenge hypothesis" (PDF). Neuroscience and Biobehavioral Reviews. 30 (3): 319–45. doi:10.1016/j.neubiorev.2004.12.007. PMID 16483890. S2CID 26405251. Archived from the original (PDF) on January 9, 2016.
- Ellis L, Hoskin AW (2015). "The evolutionary neuroandrogenic theory of criminal behavior expanded". Aggression and Violent Behavior. 24: 61–74. doi:10.1016/j.avb.2015.05.002.
- Hoskin AW, Ellis L (2015). "Fetal Testosterone and Criminality: Test of Evolutionary Neuroandrogenic Theory". Criminology. 53 (1): 54–73. doi:10.1111/1745-9125.12056.
- Perciavalle V, Di Corrado D, Petralia MC, Gurrisi L, Massimino S, Coco M (June 2013). "The second-to-fourth digit ratio correlates with aggressive behavior in professional soccer players". Molecular Medicine Reports. 7 (6): 1733–38. doi:10.3892/mmr.2013.1426. PMC 3694562. PMID 23588344.
- Bailey AA, Hurd PL (March 2005). "Finger length ratio (2D:4D) correlates with physical aggression in men but not in women". Biological Psychology. 68 (3): 215–22. doi:10.1016/j.biopsycho.2004.05.001. PMID 15620791. S2CID 16606349. Lay summary – LiveScience (March 2, 2005).
- Benderlioglu Z, Nelson RJ (December 2004). "Digit length ratios predict reactive aggression in women, but not in men". Hormones and Behavior. 46 (5): 558–64. doi:10.1016/j.yhbeh.2004.06.004. PMID 15555497. S2CID 17464657.
- Liu J, Portnoy J, Raine A (August 2012). "Association between a marker for prenatal testosterone exposure and externalizing behavior problems in children". Development and Psychopathology. 24 (3): 771–82. doi:10.1017/S0954579412000363. PMC 4247331. PMID 22781854.
- Butovskaya M, Burkova V, Karelin D, Fink B (October 1, 2015). "Digit ratio (2D:4D), aggression, and dominance in the Hadza and the Datoga of Tanzania". American Journal of Human Biology. 27 (5): 620–27. doi:10.1002/ajhb.22718. PMID 25824265. S2CID 205303673.
- Joyce CW, Kelly JC, Chan JC, Colgan G, O'Briain D, Mc Cabe JP, Curtin W (November 2013). "Second to fourth digit ratio confirms aggressive tendencies in patients with boxers fractures". Injury. 44 (11): 1636–39. doi:10.1016/j.injury.2013.07.018. PMID 23972912.
- Carré JM, Olmstead NA (February 2015). "Social neuroendocrinology of human aggression: examining the role of competition-induced testosterone dynamics" (PDF). Neuroscience. 286: 171–86. doi:10.1016/j.neuroscience.2014.11.029. PMID 25463514. S2CID 32112035.
- Klinesmith J, Kasser T, McAndrew FT (July 2006). "Guns, testosterone, and aggression: an experimental test of a mediational hypothesis". Psychological Science. 17 (7): 568–71. doi:10.1111/j.1467-9280.2006.01745.x. PMID 16866740. S2CID 33952211.
- Mcandrew FT (2009). "The Interacting Roles of Testosterone and Challenges to Status in Human Male Aggression" (PDF). Aggression and Violent Behavior. 14 (5): 330–335. doi:10.1016/j.avb.2009.04.006.
- Weierstall R, Moran J, Giebel G, Elbert T (May 1, 2014). "Testosterone reactivity and identification with a perpetrator or a victim in a story are associated with attraction to violence-related cues". International Journal of Law and Psychiatry. 37 (3): 304–12. doi:10.1016/j.ijlp.2013.11.016. PMID 24367977.
- Nguyen TV, McCracken JT, Albaugh MD, Botteron KN, Hudziak JJ, Ducharme S (January 2016). "A testosterone-related structural brain phenotype predicts aggressive behavior from childhood to adulthood". Psychoneuroendocrinology. 63: 109–18. doi:10.1016/j.psyneuen.2015.09.021. PMC 4695305. PMID 26431805.
- Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E (2010). "Prejudice and truth about the effect of testosterone on human bargaining behaviour". Nature. 463 (7279): 356–59. Bibcode:2010Natur.463..356E. doi:10.1038/nature08711. PMID 19997098. S2CID 1305527.
- van Honk J, Montoya ER, Bos PA, van Vugt M, Terburg D (May 2012). "New evidence on testosterone and cooperation". Nature. 485 (7399): E4–5, discussion E5–6. Bibcode:2012Natur.485E...4V. doi:10.1038/nature11136. PMID 22622587. S2CID 4383859.
- Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E (2012). "Eisenegger et al. reply". Nature. 485 (7399): E5–E6. Bibcode:2012Natur.485E...5E. doi:10.1038/nature11137. S2CID 4413138.
- Zak PJ, Kurzban R, Ahmadi S, Swerdloff RS, Park J, Efremidze L, Redwine K, Morgan K, Matzner W (January 1, 2009). "Testosterone administration decreases generosity in the ultimatum game". PLOS ONE. 4 (12): e8330. Bibcode:2009PLoSO...4.8330Z. doi:10.1371/journal.pone.0008330. PMC 2789942. PMID 20016825.
- McGinnis MY (December 2004). "Anabolic androgenic steroids and aggression: studies using animal models". Annals of the New York Academy of Sciences. 1036: 399–415. Bibcode:2004NYASA1036..399M. doi:10.1196/annals.1330.024. PMID 15817752. S2CID 36368056.
- von der PB, Sarkola T, Seppa K, Eriksson CJ (September 2002). "Testosterone, 5 alpha-dihydrotestosterone and cortisol in men with and without alcohol-related aggression". Journal of Studies on Alcohol. 63 (5): 518–26. doi:10.15288/jsa.2002.63.518. PMID 12380846.
- Goldman D, Lappalainen J, Ozaki N. Direct analysis of candidate genes in impulsive disorders. In: Bock G, Goode J, eds. Genetics of Criminal and Antisocial Behaviour. Ciba Foundation Symposium 194. Chichester: John Wiley & Sons; 1996.
- Coccaro E (1996). "Neurotransmitter correlates of impulsive aggression in humans. In: Ferris C, Grisso T, eds. Understanding Aggressive Behaviour inn Children". Annals of the New York Academy of Sciences. 794 (1): 82–89. Bibcode:1996NYASA.794...82C. doi:10.1111/j.1749-6632.1996.tb32511.x. PMID 8853594. S2CID 33226665.
- Finkelstein JW, Susman EJ, Chinchilli VM, Kunselman SJ, D'Arcangelo MR, Schwab J, Demers LM, Liben LS, Lookingbill G, Kulin HE (1997). "Estrogen or testosterone increases self-reported aggressive behaviors in hypogonadal adolescents". The Journal of Clinical Endocrinology & Metabolism. 82 (8): 2433–38. doi:10.1210/jcem.82.8.4165. PMID 9253313.
- Soma KK, Scotti MA, Newman AE, Charlier TD, Demas GE (October 2008). "Novel mechanisms for neuroendocrine regulation of aggression". Frontiers in Neuroendocrinology. 29 (4): 476–89. doi:10.1016/j.yfrne.2007.12.003. PMID 18280561. S2CID 32650274.
- Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC (2000). "Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows". Journal of Comparative Physiology A. 186 (7–8): 759–69. doi:10.1007/s003590000129. PMID 11016791. S2CID 23990605.
- McGinnis MY, Lumia AR, Breuer ME, Possidente B (February 2002). "Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids". Hormones and Behavior. 41 (1): 101–10. doi:10.1006/hbeh.2001.1742. PMID 11863388. S2CID 29969145.
- Sapolsky RM (2018). "Doubled-Edged Swords in the Biology of Conflict". Frontiers in Psychology. 9: 2625. doi:10.3389/fpsyg.2018.02625. PMC 6306482. PMID 30619017.
- Sapolsky RM (1998). The trouble with testosterone. New York: Simon and Schuster. pp. 153–55. ISBN 978-0-684-83891-5.
- Eisenegger C, Haushofer J, Fehr E (June 2011). "The role of testosterone in social interaction". Trends in Cognitive Sciences. 15 (6): 263–71. doi:10.1016/j.tics.2011.04.008. PMID 21616702. S2CID 9554219.
- Wilson JD (September 2001). "Androgens, androgen receptors, and male gender role behavior". Review. Hormones and Behavior. 40 (2): 358–66. doi:10.1006/hbeh.2001.1684. PMID 11534997. S2CID 20480423.
- Cosgrove KP, Mazure CM, Staley JK (October 2007). "Evolving knowledge of sex differences in brain structure, function, and chemistry". Biological Psychiatry. 62 (8): 847–55. doi:10.1016/j.biopsych.2007.03.001. PMC 2711771. PMID 17544382.
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B (July 2003). "Marked loss of myelinated nerve fibers in the human brain with age". The Journal of Comparative Neurology. 462 (2): 144–52. doi:10.1002/cne.10714. PMID 12794739. S2CID 35293796.
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R (July 1996). "The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men". The New England Journal of Medicine. 335 (1): 1–7. doi:10.1056/NEJM199607043350101. PMID 8637535.
- "Testosterone Affects Some Women's Career Choices". NPR. August 28, 2009.
- Pike CJ, Rosario ER, Nguyen TV (April 2006). "Androgens, aging, and Alzheimer's disease". Endocrine. 29 (2): 233–41. doi:10.1385/ENDO:29:2:233. PMID 16785599. S2CID 13852805.
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ (September 2004). "Age-related testosterone depletion and the development of Alzheimer disease". JAMA. 292 (12): 1431–32. doi:10.1001/jama.292.12.1431-b. PMID 15383512.
- Hogervorst E, Bandelow S, Combrinck M, Smith AD (2004). "Low free testosterone is an independent risk factor for Alzheimer's disease". Experimental Gerontology. 39 (11–12): 1633–39. doi:10.1016/j.exger.2004.06.019. PMID 15582279. S2CID 24803152.
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM (January 2004). "Free testosterone and risk for Alzheimer disease in older men". Neurology. 62 (2): 188–93. doi:10.1212/WNL.62.2.188. PMID 14745052. S2CID 10302839.
- Moffat SD, Hampson E (April 1996). "A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference". Psychoneuroendocrinology. 21 (3): 323–37. doi:10.1016/0306-4530(95)00051-8. PMID 8817730. S2CID 7135870.
- Bianchi VE (January 2019). "The Anti-Inflammatory Effects of Testosterone". Journal of the Endocrine Society. 3 (1): 91–107. doi:10.1210/js.2018-00186. PMC 6299269. PMID 30582096.
- Krysiak R, Kowalcze K, Okopień B (October 2019). "The effect of testosterone on thyroid autoimmunity in euthyroid men with Hashimoto's thyroiditis and low testosterone levels". Journal of Clinical Pharmacy and Therapeutics. 44 (5): 742–749. doi:10.1111/jcpt.12987. PMID 31183891. S2CID 184487697.
- "List of Gender Dysphoria Medications (6 Compared)". Drugs.com. Retrieved May 6, 2020.
- Myers JB, Meacham RB (2003). "Androgen replacement therapy in the aging male". Reviews in Urology. 5 (4): 216–26. PMC 1508369. PMID 16985841.
- Staff (March 3, 2015). "Testosterone Products: Drug Safety Communication – FDA Cautions About Using Testosterone Products for Low Testosterone Due to Aging; Requires Labeling Change to Inform of Possible Increased Risk of Heart Attack And Stroke". FDA. Retrieved March 5, 2015.
- "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- Qaseem A, Horwitch CA, Vijan S, Etxeandia-Ikobaltzeta I, Kansagara D (January 2020). "Testosterone Treatment in Adult Men With Age-Related Low Testosterone: A Clinical Guideline From the American College of Physicians". Annals of Internal Medicine. 172 (2): 126–133. doi:10.7326/M19-0882. PMID 31905405.
- Parry NM (January 7, 2020). "New Guideline for Testosterone Treatment in Men With 'Low T'". Medscape.com. Retrieved January 7, 2020.
- Hiipakka RA, Liao S (October 1998). "Molecular mechanism of androgen action". Trends in Endocrinology and Metabolism. 9 (8): 317–24. doi:10.1016/S1043-2760(98)00081-2. PMID 18406296. S2CID 23385663.
- McPhaul MJ, Young M (September 2001). "Complexities of androgen action". Journal of the American Academy of Dermatology. 45 (3 Suppl): S87–94. doi:10.1067/mjd.2001.117429. PMID 11511858.
- Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC (2010). "Molecular cell biology of androgen receptor signalling". Int. J. Biochem. Cell Biol. 42 (6): 813–27. doi:10.1016/j.biocel.2009.11.013. PMID 19931639.
- Wang C, Liu Y, Cao JM (2014). "G protein-coupled receptors: extranuclear mediators for the non-genomic actions of steroids". Int J Mol Sci. 15 (9): 15412–25. doi:10.3390/ijms150915412. PMC 4200746. PMID 25257522.
- Lang F, Alevizopoulos K, Stournaras C (2013). "Targeting membrane androgen receptors in tumors". Expert Opin. Ther. Targets. 17 (8): 951–63. doi:10.1517/14728222.2013.806491. PMID 23746222. S2CID 23918273.
- Breiner M, Romalo G, Schweikert HU (August 1986). "Inhibition of androgen receptor binding by natural and synthetic steroids in cultured human genital skin fibroblasts". Klinische Wochenschrift. 64 (16): 732–37. doi:10.1007/BF01734339. PMID 3762019. S2CID 34846760.
- Kelly MJ, Qiu J, Rønnekleiv OK (January 1, 2005). Estrogen signaling in the hypothalamus. Vitamins & Hormones. 71. pp. 123–45. doi:10.1016/S0083-6729(05)71005-0. ISBN 9780127098715. PMID 16112267.
- McCarthy MM (2008). "Estradiol and the developing brain". Physiological Reviews. 88 (1): 91–124. doi:10.1152/physrev.00010.2007. PMC 2754262. PMID 18195084.
- Kohtz AS, Frye CA (2012). "Dissociating behavioral, autonomic, and neuroendocrine effects of androgen steroids in animal models". Psychiatric Disorders. Methods in Molecular Biology. 829. pp. 397–431. doi:10.1007/978-1-61779-458-2_26. ISBN 978-1-61779-457-5. PMID 22231829.
- Prough RA, Clark BJ, Klinge CM (2016). "Novel mechanisms for DHEA action". J. Mol. Endocrinol. 56 (3): R139–55. doi:10.1530/JME-16-0013. PMID 26908835.
- Lazaridis I, Charalampopoulos I, Alexaki VI, Avlonitis N, Pediaditakis I, Efstathopoulos P, Calogeropoulou T, Castanas E, Gravanis A (2011). "Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis". PLOS Biol. 9 (4): e1001051. doi:10.1371/journal.pbio.1001051. PMC 3082517. PMID 21541365.
- Gravanis A, Calogeropoulou T, Panoutsakopoulou V, Thermos K, Neophytou C, Charalampopoulos I (2012). "Neurosteroids and microneurotrophins signal through NGF receptors to induce prosurvival signaling in neuronal cells". Sci Signal. 5 (246): pt8. doi:10.1126/scisignal.2003387. PMID 23074265. S2CID 26914550.
- Albayrak Y, Hashimoto K (2017). "Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders". Sigma Receptors: Their Role in Disease and as Therapeutic Targets. Advances in Experimental Medicine and Biology. 964. pp. 153–161. doi:10.1007/978-3-319-50174-1_11. ISBN 978-3-319-50172-7. PMID 28315270.
- Regitz-Zagrosek V (October 2, 2012). Sex and Gender Differences in Pharmacology. Springer Science & Business Media. pp. 245–. ISBN 978-3-642-30725-6.
- Häggström M, Richfield D (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005.
- Waterman MR, Keeney DS (1992). "Genes involved in androgen biosynthesis and the male phenotype". Hormone Research. 38 (5–6): 217–21. doi:10.1159/000182546. PMID 1307739.
- Zuber MX, Simpson ER, Waterman MR (December 1986). "Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells". Science. 234 (4781): 1258–61. Bibcode:1986Sci...234.1258Z. doi:10.1126/science.3535074. PMID 3535074.
- Zouboulis CC, Degitz K (2004). "Androgen action on human skin – from basic research to clinical significance". Experimental Dermatology. 13 Suppl 4 (s4): 5–10. doi:10.1111/j.1600-0625.2004.00255.x. PMID 15507105. S2CID 34863608.
- Brooks RV (November 1975). "Androgens". Clinics in Endocrinology and Metabolism. 4 (3): 503–20. doi:10.1016/S0300-595X(75)80045-4. PMID 58744.
- Payne AH, O'Shaughnessy P (1996). "Structure, function, and regulation of steroidogenic enzymes in the Leydig cell". In Payne AH, Hardy MP, Russell LD (eds.). Leydig Cell. Vienna [Il]: Cache River Press. pp. 260–85. ISBN 978-0-9627422-7-9.
- Swerdloff RS, Wang C, Bhasin S (April 1992). "Developments in the control of testicular function". Baillière's Clinical Endocrinology and Metabolism. 6 (2): 451–83. doi:10.1016/S0950-351X(05)80158-2. PMID 1377467.
- Liverman CT, Blazer DG, Institute of Medicine (US) Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy (January 1, 2004). "Introduction". Testosterone and Aging: Clinical Research Directions. National Academies Press (US). doi:10.17226/10852. ISBN 978-0-309-09063-6. PMID 25009850 – via www.ncbi.nlm.nih.gov.
- Huhtaniemi I (2014). "Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment". Asian Journal of Andrology. 16 (2): 192–202. doi:10.4103/1008-682X.122336. PMC 3955328. PMID 24407185.
- Huhtaniemi IT (2014). "Andropause--lessons from the European Male Ageing Study". Annales d'Endocrinologie. 75 (2): 128–31. doi:10.1016/j.ando.2014.03.005. PMID 24793989.
- Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM (2010). "Testosterone physiology in resistance exercise and training: the up-stream regulatory elements". Sports Medicine. 40 (12): 1037–53. doi:10.2165/11536910-000000000-00000. PMID 21058750. S2CID 11683565.
- Hulmi JJ, Ahtiainen JP, Selänne H, Volek JS, Häkkinen K, Kovanen V, Mero AA (May 2008). "Androgen receptors and testosterone in men—effects of protein ingestion, resistance exercise and fiber type". The Journal of Steroid Biochemistry and Molecular Biology. 110 (1–2): 130–37. doi:10.1016/j.jsbmb.2008.03.030. PMID 18455389. S2CID 26280370.
- Hackney AC, Moore AW, Brownlee KK (2005). "Testosterone and endurance exercise: development of the "exercise-hypogonadal male condition"". Acta Physiologica Hungarica. 92 (2): 121–37. doi:10.1556/APhysiol.92.2005.2.3. PMID 16268050.
- Livera G, Rouiller-Fabre V, Pairault C, Levacher C, Habert R (August 2002). "Regulation and perturbation of testicular functions by vitamin A". Reproduction. 124 (2): 173–80. doi:10.1530/rep.0.1240173. PMID 12141930.
- Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A (March 2011). "Effect of vitamin D supplementation on testosterone levels in men". Hormone and Metabolic Research = Hormon- und Stoffwechselforschung = Hormones et Métabolisme. 43 (3): 223–25. doi:10.1055/s-0030-1269854. PMID 21154195.
- Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ (May 1996). "Zinc status and serum testosterone levels of healthy adults". Nutrition. 12 (5): 344–48. CiteSeerX 10.1.1.551.4971. doi:10.1016/S0899-9007(96)80058-X. PMID 8875519.
- Koehler K, Parr MK, Geyer H, Mester J, Schänzer W (January 2009). "Serum testosterone and urinary excretion of steroid hormone metabolites after administration of a high-dose zinc supplement". European Journal of Clinical Nutrition. 63 (1): 65–70. doi:10.1038/sj.ejcn.1602899. PMID 17882141.
- Håkonsen LB, Thulstrup AM, Aggerholm AS, Olsen J, Bonde JP, Andersen CY, Bungum M, Ernst EH, Hansen ML, Ernst EH, Ramlau-Hansen CH (2011). "Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men". Reproductive Health. 8 (1): 24. doi:10.1186/1742-4755-8-24. PMC 3177768. PMID 21849026.
- MacDonald AA, Herbison GP, Showell M, Farquhar CM (2010). "The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis". Human Reproduction Update. 16 (3): 293–311. doi:10.1093/humupd/dmp047. PMID 19889752.
- Andersen ML, Tufik S (October 2008). "The effects of testosterone on sleep and sleep-disordered breathing in men: its bidirectional interaction with erectile function". Sleep Medicine Reviews. 12 (5): 365–79. doi:10.1016/j.smrv.2007.12.003. PMID 18519168.
- Schultheiss OC, Campbell KL, McClelland DC (December 1999). "Implicit power motivation moderates men's testosterone responses to imagined and real dominance success". Hormones and Behavior. 36 (3): 234–41. doi:10.1006/hbeh.1999.1542. PMID 10603287. S2CID 6002474.
- Akdoğan M, Tamer MN, Cüre E, Cüre MC, Köroğlu BK, Delibaş N (May 2007). "Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism". Phytotherapy Research. 21 (5): 444–47. doi:10.1002/ptr.2074. PMID 17310494. S2CID 21961390.
- Kumar V, Kural MR, Pereira BM, Roy P (December 2008). "Spearmint induced hypothalamic oxidative stress and testicular anti-androgenicity in male rats - altered levels of gene expression, enzymes and hormones". Food and Chemical Toxicology. 46 (12): 3563–70. doi:10.1016/j.fct.2008.08.027. PMID 18804513.
- Grant P (February 2010). "Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial". Phytotherapy Research. 24 (2): 186–88. doi:10.1002/ptr.2900. PMID 19585478. S2CID 206425734.
- Armanini D, Fiore C, Mattarello MJ, Bielenberg J, Palermo M (September 2002). "History of the endocrine effects of licorice". Experimental and Clinical Endocrinology & Diabetes. 110 (6): 257–61. doi:10.1055/s-2002-34587. PMID 12373628.
- Nieschlag E, Behre HM, Nieschlag S (July 26, 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 61–. ISBN 978-1-107-01290-5.
- Cumming DC, Wall SR (November 1985). "Non-sex hormone-binding globulin-bound testosterone as a marker for hyperandrogenism". The Journal of Clinical Endocrinology & Metabolism. 61 (5): 873–6. doi:10.1210/jcem-61-5-873. PMID 4044776.
- Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1116, 1119, 1183. ISBN 978-0-7817-1750-2.
- Wecker L, Watts S, Faingold C, Dunaway G, Crespo L (April 1, 2009). Brody's Human Pharmacology. Elsevier Health Sciences. pp. 468–469. ISBN 978-0-323-07575-6.
- Penning TM (2010). "New frontiers in androgen biosynthesis and metabolism". Curr Opin Endocrinol Diabetes Obes. 17 (3): 233–9. doi:10.1097/MED.0b013e3283381a31. PMC 3206266. PMID 20186052.
- Horsky J, Presl J (December 6, 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 107–. ISBN 978-94-009-8195-9.
- Zhou S (April 6, 2016). Cytochrome P450 2D6: Structure, Function, Regulation and Polymorphism. CRC Press. pp. 242–. ISBN 978-1-4665-9788-4.
- Trager L (1977). Steroidhormone: Biosynthese, Stoffwechsel, Wirkung (in German). Springer-Verlag. p. 349. ISBN 978-0-387-08012-3.
- Randall VA (April 1994). "Role of 5 alpha-reductase in health and disease". Baillière's Clinical Endocrinology and Metabolism. 8 (2): 405–31. doi:10.1016/S0950-351X(05)80259-9. PMID 8092979.
- Meinhardt U, Mullis PE (August 2002). "The essential role of the aromatase/p450arom". Seminars in Reproductive Medicine. 20 (3): 277–84. doi:10.1055/s-2002-35374. PMID 12428207.
- Noakes DE (April 23, 2009). Arthur's Veterinary Reproduction and Obstetrics. Elsevier Health Sciences UK. pp. 695–. ISBN 978-0-7020-3990-4.
- Nieschlag E, Behre HM (April 1, 2004). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 626–. ISBN 978-1-139-45221-2.
- Parl FF (2000). Estrogens, Estrogen Receptor and Breast Cancer. IOS Press. pp. 25–. ISBN 978-0-9673355-4-4.
- Norman AW, Henry HL (July 30, 2014). Hormones. Academic Press. pp. 261–. ISBN 978-0-08-091906-5.
- Mozayani A, Raymon L (September 18, 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
- Sundaram K, Kumar N, Monder C, Bardin CW (1995). "Different patterns of metabolism determine the relative anabolic activity of 19-norandrogens". J. Steroid Biochem. Mol. Biol. 53 (1–6): 253–7. doi:10.1016/0960-0760(95)00056-6. PMID 7626464. S2CID 32619627.
- Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, Lapauw B, Fiers T, Matsumoto AM, Bhasin S (April 2017). "Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe". The Journal of Clinical Endocrinology & Metabolism. 102 (4): 1161–1173. doi:10.1210/jc.2016-2935. PMC 5460736. PMID 28324103.
- Sperling MA (April 10, 2014). Pediatric Endocrinology E-Book. Elsevier Health Sciences. pp. 488–. ISBN 978-1-4557-5973-6.
- Pauline M. Camacho (September 26, 2012). Evidence-Based Endocrinology. Lippincott Williams & Wilkins. pp. 217–. ISBN 978-1-4511-7146-4.
- Steinberger E, Ayala C, Hsi B, Smith KD, Rodriguez-Rigau LJ, Weidman ER, Reimondo GG (1998). "Utilization of commercial laboratory results in management of hyperandrogenism in women". Endocr Pract. 4 (1): 1–10. doi:10.4158/EP.4.1.1. PMID 15251757.
- Bajaj L, Berman S (January 1, 2011). Berman's Pediatric Decision Making. Elsevier Health Sciences. pp. 160–. ISBN 978-0-323-05405-8.
- Dennis M. Styne (April 25, 2016). Pediatric Endocrinology: A Clinical Handbook. Springer. pp. 191–. ISBN 978-3-319-18371-8.
- Pagana KD, Pagana TJ, Pagana TN (September 19, 2014). Mosby's Diagnostic and Laboratory Test Reference – E-Book. Elsevier Health Sciences. pp. 879–. ISBN 978-0-323-22592-2.
- Engorn B, Flerlage J (May 1, 2014). The Harriet Lane Handbook E-Book. Elsevier Health Sciences. pp. 240–. ISBN 978-0-323-11246-8.
- de Ronde W, van der Schouw YT, Pols HA, Gooren LJ, Muller M, Grobbee DE, de Jong FH (September 2006). "Calculation of bioavailable and free testosterone in men: a comparison of 5 published algorithms". Clinical Chemistry. 52 (9): 1777–84. doi:10.1373/clinchem.2005.063354. PMID 16793931.
- Hasler J, Herklotz R, Luppa PB, Diver MJ, Thevis M, Metzger J, Savoca R, Jermini F, Huber AR (January 1, 2006). "Impact of recent biochemical findings on the determination of free and bioavailable testosterone: evaluation and proposal for clinical use". LaboratoriumsMedizin. 30 (6): 492–505. doi:10.1515/JLM.2006.050.
- "RCSB PDB - 1D2S". Crystal Structure of the N-Terminal Laminin G-Like Domain of SHBG in Complex with Dihydrotestosterone.
- Berthold AA (1849). "Transplantation der Hoden" [Transplantation of testis]. Arch. Anat. Physiol. Wiss. (in German). 16: 42–6.
- Brown-Sequard CE (1889). "The effects produced on man by subcutaneous injections of liquid obtained from the testicles of animals". Lancet. 2 (3438): 105–07. doi:10.1016/S0140-6736(00)64118-1.
- Gallagher TF, Koch FC (November 1929). "The testicular hormone". J. Biol. Chem. 84 (2): 495–500.
- David KG, Dingemanse E, Freud JL (May 1935). "Über krystallinisches mannliches Hormon aus Hoden (Testosteron) wirksamer als aus harn oder aus Cholesterin bereitetes Androsteron" [On crystalline male hormone from testicles (testosterone) effective as from urine or from cholesterol]. Hoppe-Seyler's Z Physiol Chem (in German). 233 (5–6): 281–83. doi:10.1515/bchm2.1935.233.5-6.281.
- Butenandt A, Hanisch G (1935). "Umwandlung des Dehydroandrosterons in Androstendiol und Testosterone; ein Weg zur Darstellung des Testosterons aus Cholestrin" [About Testosterone. Conversion of Dehydro-androsterons into androstendiol and testosterone; a way for the structure assignment of testosterone from cholesterol]. Hoppe-Seyler's Z Physiol Chem (in German). 237 (2): 89–97. doi:10.1515/bchm2.1935.237.1-3.89.
- Freeman ER, Bloom DA, McGuire EJ (February 2001). "A brief history of testosterone". The Journal of Urology. 165 (2): 371–73. doi:10.1097/00005392-200102000-00004. PMID 11176375.
- Butenandt A, Hanisch G (1935). "Uber die Umwandlung des Dehydroandrosterons in Androstenol-(17)-one-(3) (Testosterone); um Weg zur Darstellung des Testosterons auf Cholesterin (Vorlauf Mitteilung). [The conversion of dehydroandrosterone into androstenol-(17)-one-3 (testosterone); a method for the production of testosterone from cholesterol (preliminary communication)]". Chemische Berichte (in German). 68 (9): 1859–62. doi:10.1002/cber.19350680937.
- Ruzicka L, Wettstein A (1935). "Uber die kristallinische Herstellung des Testikelhormons, Testosteron (Androsten-3-ol-17-ol) [The crystalline production of the testicle hormone, testosterone (Androsten-3-ol-17-ol)]". Helvetica Chimica Acta (in German). 18: 1264–75. doi:10.1002/hlca.193501801176.
- Hoberman JM, Yesalis CE (February 1995). "The history of synthetic testosterone". Scientific American. 272 (2): 76–81. Bibcode:1995SciAm.272b..76H. doi:10.1038/scientificamerican0295-76. PMID 7817189.
- Kenyon AT, Knowlton K, Sandiford I, Koch FC, Lotwin, G (February 1940). "A comparative study of the metabolic effects of testosterone propionate in normal men and women and in eunuchoidism". Endocrinology. 26 (1): 26–45. doi:10.1210/Endo-26-1-26.
- Schwarz S, Onken D, Schubert A (July 1999). "The steroid story of Jenapharm: from the late 1940s to the early 1970s". Steroids. 64 (7): 439–45. doi:10.1016/S0039-128X(99)00003-3. PMID 10443899. S2CID 40156824.
- de Kruif P (1945). The Male Hormone. New York: Harcourt, Brace.
- Guerriero G (2009). "Vertebrate sex steroid receptors: evolution, ligands, and neurodistribution". Annals of the New York Academy of Sciences. 1163 (1): 154–68. Bibcode:2009NYASA1163..154G. doi:10.1111/j.1749-6632.2009.04460.x. PMID 19456336. S2CID 5790990.
- Bryan MB, Scott AP, Li W (2008). "Sex steroids and their receptors in lampreys". Steroids. 73 (1): 1–12. doi:10.1016/j.steroids.2007.08.011. PMID 17931674. S2CID 33753909.
- Nelson RF (2005). An introduction to behavioral endocrinology. Sunderland, Mass: Sinauer Associates. p. 143. ISBN 978-0-87893-617-5.
- De Loof A (October 2006). "Ecdysteroids: the overlooked sex steroids of insects? Males: the black box". Insect Science. 13 (5): 325–338. doi:10.1111/j.1744-7917.2006.00101.x.
- Mechoulam R, Brueggemeier RW, Denlinger DL (September 1984). "Estrogens in insects". Journal Cellular and Molecular Life Sciences. 40 (9): 942–44. doi:10.1007/BF01946450. S2CID 31950471.
Further reading
- Fargo KN, Pak TR, Foecking EM, Jones KJ (2010). "Molecular Biology of Androgen Action: Perspectives on Neuroprotective and Neurotherapeutic Effects.". In Pfaff DW, Etgen AM (eds.). Molecular Mechanisms of Hormone Actions on Behavior. Elsevier Inc. pp. 1219–1246. doi:10.1016/B978-008088783-8.00036-X. ISBN 978-0-12-374939-0.
- Dowd NE (2013). "Sperm, testosterone, masculinities and fatherhood". Nevada Law Journal. 13 (2): 8.