Tetraamminecopper(II) sulfate
Tetraamminecopper(II) sulfate is the salt with the formula [Cu(NH3)4]SO4·H2O. This dark blue to purple solid is a salt of the metal complex [Cu(NH3)4(H2O)]2+. It is closely related to Schweizer's reagent, which is used for the production of cellulose fibers in the production of rayon. It is used to print fabrics, used as a pesticide and to make other copper compounds like copper nano-powder.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Tetraamminecopper(II) sulfate monohydrate | |
| Other names
cuprammonium(II) sulfate; cupric sulfate, ammoniated | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.155.305 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| [Cu(NH3)4]SO4·H2O | |
| Molar mass | 245.79 g/mol (monohydrate) |
| Appearance | dark blue-purple solution or crystals |
| Odor | Ammonia |
| Density | 1.81 g/cm3 |
| Boiling point | 330 °C (626 °F; 603 K)[1] |
| 18.5 g/100 g (21.5 °C)[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
This compound can be prepared by adding concentrated solution of ammonia to a saturated aqueous solution of copper sulfate pentahydrate followed by precipitation of the product with ethanol or isopropanol.[2]
- 4 NH3 + CuSO4·5H2O →[Cu(NH3)4]SO4·H2O+ 4 H2O
Chemical reaction and solubility
The deep blue crystalline solid tends to hydrolyse and evolve (release) ammonia upon standing in air.[2] It is fairly soluble in water. The brilliant dark blue-violet color of tetraamminecopper(II) sulfate solution is due to presence of [Cu(NH3)4]2+. Often, the dark blue-violet color is used as a positive test to verify the presence of Cu2+ in a solution.
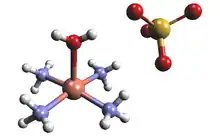
Structure and properties
The solid state salt of tetraamminecopper(II) sulfate contains the complex ion [Cu(NH3)4H2O]2+, which has a square pyramidal molecular geometry. The bond length between the atoms in the crystal are measured using X-ray crystallography; the Cu-N and Cu-O distances are about 210 and 233 pm, respectively.[3] Cu(NH3)The correct concentrations of ammonia and copper sulfate solution needed to synthesize the complex can be determined by colorimetry. The combination of the correct concentrations will produce the highest absorbance read out on the colorimeter and as a result the formula of the complex can be verified.
Corrosion
The characteristic deep blue colour of the tetraammine complex is found in brass and copper alloys where attack from ammonia has occurred, leading to cracking. The problem was first found in ammunition cartridge cases when they were stored near animal waste, which produced trace amounts of ammonia. This type of corrosion is known as season cracking.
Uses
The closely related Schweizer's reagent is used for the production of cuprammonium rayon.
Most pesticides contain ammonium sulfate. Ammonium sulfate is used as an agricultural spray adjuvant for water-soluble insecticides, herbicides, and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2, 4-D (amine), glyphosate, and glufosinate herbicides.[1] The brilliant dark blue-violet color and a good solubility make tetraammine copper (II) sulfate an excellent chemical to dye fabrics. Some of the recent research and development for copper include various studies of tetraaminecopper (II) sulfate. One such research is "Chemical reduction method for preparing copper nano-powder with high purity using sodium hypophosphite as reductant", which uses tetraaminecopper (II) sulfate.
Other property
The magnetic and thermal properties of tetraammine copper (II) sulfate monohydrate have been extensively studied and have been interpreted as those of a substance containing a magnetic linear chain structure. In 1967, Saito & Kanda conducted proton nuclear magnetic resonance studies on this material in the paramagnetic and ordered state. Unfortunately, the lack of proton positions prevented a quantitative interpretation of these data. Since the early crystallographic study by Mazzi (1955) reported a structure based on projected data and indicated an unusual coordination about the copper ion, a detail structure determination was considered necessary for any future quantitative interpretation of the observed resonance data.[4]

References
- American Elements – The material science company; tetraammine copper(II) sulfate monohydrate; CAS 10380-29-7
- Editor G.Brauer "Tetraamminecopper (II) Sulfate" Handbook of Preparative Inorganic Chemistry, 2nd Ed., Academic Press, 1965, New York. Vol. 2. p. 1021.
- Morosin "The Crystal Structures of Copper Tetraammine Complexes. A. and " Acta Crystallographica 1969, vol. B25, pp. 19–30. doi:10.1107/S0567740869001725
- Li, Yudan; Wang, Wenjie; He, Chuan.; Chemical reduction method for preparing copper nanopowder with high purity using sodium hypophosphite as reductant, Faming Zhuanli Shenqing, December 4, 2013, CN 103418801