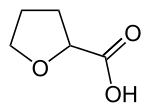
Tetrahydro-2-furoic acid
Tetrahydro-2-furoic acid is an organic compound with the formula HO2CC4H7O. It is a colorless oil. Tetrahydro-2-furoic acid is a useful pharmaceutical intermediate relevant to the production of several drugs, including Terazosin for the treatment of prostate enlargement and hypertension.[1][2] or high boiling liquid,[3]
 | |
| Names | |
|---|---|
| IUPAC name
Tetrahydro-2-furancarboxylic acid | |
| Other names
Tetrahydro-2-furoic acid; Tetrahydrofuran-2-carboxylic acid; Tetrahydrofuroic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.122.132 |
| MeSH | C063698 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C5H8O3 | |
| Molar mass | 116.116 g·mol−1 |
| Appearance | colorlesss oil |
| Density | 1.262 g/cm3 @ 20 °C |
| Melting point | 21 °C (70 °F; 294 K) |
| Boiling point | 135 °C (275 °F; 408 K) 20 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Furoic acid is reduced to tetrahydro-2-furoic acid, as originally reported in 1913 by Wienhaus.[4] Tetrahydro-2-furoic acid has been prepared via selective hydrogenation of 2-furoic acid over a bimetallic catalyst of palladium-nickel supported on alumina.[5]
Enantioselective heterogeneous hydrogenation of furoic acid to chiral tetrahydro-2-furoic acid proceeds in the presence of cinchonidine-modified alumina supported palladium catalyst in 95% yield and 32% enantiomeric excess.[6] Similarly, homogeneous hydrogenation to chiral tetrahydro-2-furoic acid proceeds quantitatively with 24-27% enantiomeric excess in methanol solution employing a chiral, ferrocene-phosphine catalyst.[7]
Applications
Pharmaceuticals
Reaction of tetrahydro-2-furoic acid with the hydrochloride salt of 3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]-propanenitrile provided alfuzosin, a drug for the treatment of benign prostatic hyperplasia (BPH).[8]
A key intermediate to faropenem, an antibiotic for the treatment of acute bacterial sinusitis, chronic bronchitis and pneumonia has been prepared from tetrahydro-2-furoic acid via a process including chiral resolution and chlorination.[9]
Tecadenoson is another example of a drug made using tetrahydro-2-furoic acid.
References
- Wen-Chih Chou, Ming-Chen Chou, Yann-Yu Lu and Shyh-Fong Chen, "Preparation of N-acylalkylenediamines as precursors of antihypertensive quinazolines", US Patent (2001), 6313293(B1).
- Franco Codignola and Mario Piacenza, "A process for the production of polyurethane resins", Italian Patent (1947), ES179144 (A1).
- Raymond Paul; Tchelitcheff, Serge (1952). "Action of oragno-sodium derivatives on vinyl ethers". Compt. Rend. 235: 1226–8.
- Heinrich Wienhaus; Sorge, Hermann (1913). "Reduction of Pyromucic Acid". Berichte der Deutschen Chemischen Gesellschaft. 46: 1927–31. doi:10.1002/cber.191304602107.
- Zhe-qi Li; Ding, Yun-jie; Jiang, Wen-feng (2005). "Study on the performance of α-furanoic acid hydrogenation over Pd-Ni/Al2O3 catalysts under mild conditions". Fenzi Cuihua. 19 (2): 131–135.
- Mihaela Maris; Huck, Wolf-Rudiger; Mallat, Tamas; Baiker, Alfons (2003). "Palladium-catalyzed asymmetric hydrogenation of furan carboxylic acids". Journal of Catalysis. 219 (1): 52–58. doi:10.1016/s0021-9517(03)00184-2.
- Martin Studer; Wedemeyer-Exl, Christina; Spindler, Felix; Blaser, Hans-Ulrich (2000). "Enantioselective homogeneous hydrogenation of monosubstituted pyridines and furans". Monatshefte für Chemie. 131 (12): 1335–1343. doi:10.1007/s007060070013.
- Uday Rajaram Bapat , Jose Paul Potams, Narasimhan Subramanian and Jon Valgeirsson, "Process for the preparation of alfuzosin and salts thereof ", PCT Int. Appl. (2008), 2008152514.
- Hongna Han; Jin, Jie; Liu, Jun (2001). "Synthesis of key intermediate of faropenem: (3S,4R)-3-[1- ethyl]-4-(tetrahydrofuran-2- carbonylmercapto)-2-azetidinone". Shenyang Yaoke Daxue Xuebao. 18 (1): 20–22.