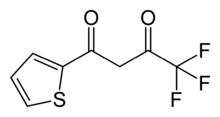
Thenoyltrifluoroacetone
Thenoyltrifluoroacetone, C8H5F3O2S, is a chemical compound used pharmacologically as a chelating agent. It is an inhibitor of cellular respiration by blocking the respiratory chain at complex II.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione | |
| Other names
2-thenoyltrifluoroacetone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.743 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H5F3O2S | |
| Molar mass | 222.18 g mol−1 |
| Appearance | fine, slightly yellow crystals |
| Melting point | 40 to 44 °C (104 to 111 °F; 313 to 317 K) |
| Boiling point | 96 to 98 °C (205 to 208 °F; 369 to 371 K) 8 mmHg |
| Hazards | |
| Main hazards | Xi |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S26 S27 S28 S29 S30 S33 S35 S36 |
| Flash point | 12 °C (54 °F; 285 K) (closed cup) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Perhaps the first report of TTFA as an inhibitor of respiration was by A. L. Tappel in 1960.[2] Tappel had the (erroneous) idea that inhibitors like antimycin and alkyl hydroxyquinoline-N-oxide might work by chelating iron in the hydrophobic milieu of respiratory membrane proteins, so he tested a series of hydrophobic chelating agents. TTFA was a potent inhibitor, but not because of its chelating ability. TTFA binds at the quinone reduction site in Complex II, preventing ubiquinone from binding. The first x-ray structure of Complex II showing how TTFA binds, 1ZP0, was published in 2005 .[3]
References
- Sigma-Aldrich product page
- Tappel (July 1960). "Inhibition of electron transport by antimycin A, alkyl hydroxy naphthoquinones and metal coordination compounds". Biochem. Pharmacol. 3 (4): 289–96. doi:10.1016/0006-2952(60)90094-0. PMID 13836892.
- Sun, Fei; Huo, Xia; Zhai, Yujia; Wang, Aojin; Xu, Jianxing; Su, Dan; Bartlam, Mark; Rao, Zihe (2005). "Crystal Structure of Mitochondrial Respiratory Membrane Protein Complex II". Cell. 121 (7): 1043–1047. doi:10.1016/j.cell.2005.05.025. PMID 15989954. S2CID 16697879.
| Wikimedia Commons has media related to thenoyltrifluoroacetone. |