Thermal barrier coating
Thermal barrier coatings (TBCs) are advanced materials systems usually applied to metallic surfaces operating at elevated temperatures, such as gas turbine or aero-engine parts, as a form of exhaust heat management. These 100 μm to 2 mm thick coatings of thermally insulating materials serve to insulate components from large and prolonged heat loads and can sustain an appreciable temperature difference between the load-bearing alloys and the coating surface.[1] In doing so, these coatings can allow for higher operating temperatures while limiting the thermal exposure of structural components, extending part life by reducing oxidation and thermal fatigue. In conjunction with active film cooling, TBCs permit working fluid temperatures higher than the melting point of the metal airfoil in some turbine applications. Due to increasing demand for more efficient engines running at higher temperatures with better durability/lifetime and thinner coatings to reduce parasitic mass for rotating/moving components, there is significant motivation to develop new and advanced TBCs. The material requirements of TBCs are similar to those of heat shields, although in the latter application emissivity tends to be of greater importance.
.jpg.webp)
Structure
An effective TBC needs to meet certain requirements to perform well in aggressive thermo-mechanical environments.[2] To deal with thermal expansion stresses during heating and cooling, adequate porosity is needed, as well as appropriate matching of thermal expansion coefficients with the metal surface that the TBC is coating. Phase stability is required to prevent significant volume changes (which occur during phase changes), which would cause the coating to crack or spall. In air-breathing engines, oxidation resistance is necessary, as well as decent mechanical properties for rotating/moving parts or parts in contact. Therefore, general requirements for an effective TBC can be summarize as needing: 1) a high melting point. 2) no phase transformation between room temperature and operating temperature. 3) low thermal conductivity. 4) chemical inertness. 5) similar thermal expansion match with the metallic substrate. 6) good adherence to the substrate. 7) low sintering rate for a porous microstructure. These requirements severely limit the number of materials that can be used, with ceramic materials usually being able to satisfy the required properties.[3]
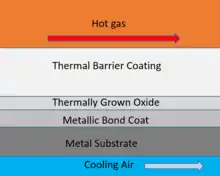
Thermal barrier coatings typically consist of four layers: the metal substrate, metallic bond coat, thermally-grown oxide (TGO), and ceramic topcoat. The ceramic topcoat is typically composed of yttria-stabilized zirconia (YSZ), which has very low conductivity while remaining stable at the nominal operating temperatures typically seen in TBC applications. This ceramic layer creates the largest thermal gradient of the TBC and keeps the lower layers at a lower temperature than the surface. However, above 1200 °C, YSZ suffers from unfavorable phase transformations, changing from t'-tetragonal to tetragonal to cubic to monoclinic. Such phase transformations lead to crack formation within the top coating. Recent efforts to develop an alternative to the YSZ ceramic topcoat have identified many novel ceramics (e.g., rare earth zirconates) exhibiting superior performance at temperatures above 1200 °C, but with inferior fracture toughness compared to that of YSZ. In addition, such zirconates may have a high concentration of oxygen-ion vacancies, which may facilitate oxygen transport and exacerbate the formation of the TGO. With a thick enough TGO, spalling of the coating may occur, which is a catastrophic mode of failure for TBCs. The use of such coatings would require additional coatings that are more oxidation resistant, such as alumina or mullite.[4]
The bond coat is an oxidation-resistant metallic layer which is deposited directly on top of the metal substrate. It is typically 75-150 μm thick and made of a NiCrAlY or NiCoCrAlY alloy, though other bond coats made of Ni and Pt aluminides also exist. The primary purpose of the bond coat is to protect the metal substrate from oxidation and corrosion, particularly from oxygen and corrosive elements that pass through the porous ceramic top coat.
At peak operating conditions found in gas-turbine engines with temperatures in excess of 700 °C, oxidation of the bond-coat leads to the formation of a thermally-grown oxide (TGO) layer. Formation of the TGO layer is inevitable for many high-temperature applications, so thermal barrier coatings are often designed so that the TGO layer grows slowly and uniformly. Such a TGO will have a structure that has a low diffusivity for oxygen, so that further growth is controlled by diffusion of metal from the bond-coat rather than the diffusion of oxygen from the top-coat.[5]
The TBC can also be locally modified at the interface between the bond coat and the thermally grown oxide so that it acts as a thermographic phosphor, which allows for remote temperature measurement
Failure
TBCs fail through various degradation modes that include mechanical rumpling of the bond coat during thermal cyclic exposure (especially coatings in aircraft engines), accelerated oxidation, hot corrosion, or molten deposit degradation. There are also issues with oxidation (areas of the TBC start being stripped off) of the TBC, which reduces the life of the metal component drastically, which leads to thermal fatigue.
A key feature of all TBC components is the need for well-matched thermal expansion coefficients between all layers. Thermal barrier coatings expand and contract at different rates upon heating and cooling of the environment, so if the materials of the different layers have poorly matched thermal expansion coefficients, a strain is introduced which can lead to cracking and ultimately failure of the coating.
Cracking at the thermally-grown oxide (TGO) layer between the top coat and bond coat is the most common failure mode for gas-turbine blade coatings. TGO growth produces a stress associated with the volume expansion which persists at all temperatures. When the system is cooled, even more mismatch is introduced from the mismatch in thermal expansion coefficients. The result is very high (2-6 GPa) stresses which occur at low temperature and can produce cracking and ultimately spalling of the barrier coating. TGO formation also results in depletion of Al in the bond coat. This can lead to the formation of undesirable phases which contribute to the mismatch stresses. These processes are all accelerated by the thermal cycling that many thermal barrier coatings undergo in practice.[6]
Types[7]
YSZ
YSZ is the most widely studied and used TBC because it provides excellent performance in applications such as diesel engines and gas turbines. Additionally, it was one of the few refractory oxides that could be deposited as thick films using the then-known technology of plasma spraying.[8] As for properties, it has low thermal conductivity, high thermal expansion coefficient, and low thermal shock resistance. However, it has a fairly low operating limit of 1200°C due to phase instability, and can corrode due to its oxygen transparency.
Mullite
Mullite is a compound of alumina and silica, with the formula 3Al2O3-2SiO2. It has a low density, along with good mechanical properties, high thermal stability, low thermal conductivity, and is corrosion and oxidation resistant. However, it suffers from crystallization and volume contraction above 800°C, which leads to cracking and delamination. Therefore, this material is suitable as a zirconia alternative for applications such as diesel engines, where surface temperatures are relatively low and temperature variations across the coating may be large.
Alumina
Only α-phase Al2O3 is stable among aluminum oxides. With a high hardness and chemical inertness, but high thermal conductivity and low thermal expansion coefficient, alumina is often used as an addition to an existing TBC coating. By incorporating alumina in YSZ TBC, oxidation and corrosion resistance can be improved, as well as hardness and bond strength without significant change in the elastic modulus or toughness. One challenge with alumina is applying the coating through plasma spraying, which tends to create a variety of unstable phases, such as γ-alumina. When these phases eventually transform into the stable α-phase through thermal cycling, a significant volume change of ~15% (γ to α) follows, which can lead to microcrack formation in the coating.
CeO2 + YSZ
CeO2 (Ceria) has a higher thermal expansion coefficient and lower thermal conductivity than YSZ. Adding ceria into a YSZ coating can significantly improve the TBC performance, especially in thermal shock resistance. This is most likely due to less bond coat stress due to better insulation and a better net thermal expansion coefficient. Some negative effects of the addition of ceria include the decrease of hardness and accelerated rate of sintering of the coating (less porous).
Rare-earth zirconates
La2Zr2O7, also referred to as LZ, is an example of a rare-earth zirconate that shows potential for use as a TBC. This material is phase stable up to its melting point and can largely tolerate vacancies on any of its sublattices. Along with the ability for site-substitution with other elements, this means that thermal properties can potentially be tailored. Although it has a very low thermal conductivity compared to YSZ, it also has a low thermal expansion coefficient and low toughness.
Rare earth oxides
The mixture of rare earth oxides is readily available, cheap, and may have promise as effective TBCs. The coatings of rare earth oxides (ex: La2O3, Nb2O5, Pr2O3, CeO2 as main phases) have lower thermal conductivity and higher thermal expansion coefficients when compared to YSZ. The main challenge to overcome is the polymorphic nature of most rare earth oxides at elevated temperatures, as phase instability tends to negatively impact thermal shock resistance.
Metal-Glass Composites
A powder mixture of metal and normal glass can be plasma-sprayed in vacuum, with a suitable composition resulting in a TBC comparable to YSZ. Additionally, metal-glass composites have superior bond-coat adherence, higher thermal expansion coefficients, and no open porosity, which prevents oxidation of the bond-coat.
Uses


Automotive
Thermal barrier ceramic coatings are becoming more common in automotive applications. They are specifically designed to reduce heat loss from engine exhaust system components including exhaust manifolds, turbocharger casings, exhaust headers, downpipes and tailpipes. This process is also known as "exhaust heat management". When used under-bonnet, these have the positive effect of reducing engine bay temperatures, therefore reducing the intake air temperature.
Although most ceramic coatings are applied to metallic parts directly related to the engine exhaust system, technological advances now allow thermal barrier coatings to be applied via plasma spray onto composite materials. It is now commonplace to find ceramic-coated components in modern engines and on high-performance components in race series such as Formula 1. As well as providing thermal protection, these coatings are also used to prevent physical degradation of the composite material due to friction. This is possible because the ceramic material bonds with the composite (instead of merely sticking on the surface with paint), thereby forming a tough coating that doesn't chip or flake easily.
Although thermal barrier coatings have been applied to the insides of exhaust system components, problems have been encountered because of the difficulty in preparing the internal surface prior to coating.
Aviation
Interest in increasing the efficiency of gas turbine engines for aviation applications has prompted research into higher combustion temperatures. Turbine efficiency is strongly correlated with combustion temperature. Higher temperature combustion improves the thermodynamic efficiency of the machine, giving a more favourable ratio of work generated in relation to waste heat.[9]Thermal barrier coatings are commonly used to protect nickel-based superalloys from both melting and thermal cycling in aviation turbines. Combined with cool air flow, TBCs increase the allowable gas temperature above that of the superalloy melting point.[10]
To avoid the difficulties associated with the melting point of superalloys, many researchers are investigating ceramic-matrix composites (CMCs) as high-temperature alternatives. Generally, these are made from fiber-reinforced SiC. Rotating parts are especially good candidates for the material change due to the enormous fatigue that they endure. Not only do CMCs have better thermal properties, but they are also lighter meaning that less fuel would be needed to produce the same thrust for the lighter aircraft.[11] The material change is, however, not without consequences. At high temperatures, these CMCs are reactive with water and form gaseous silicon hydroxide compounds that corrode the CMC.
SiOH2 + H2O = SiO(OH)2
SiOH2 + 2H2O = Si(OH)4
2SiOH2 + 3H2O = Si2O(OH)6[12]
The thermodynamic data for these reactions has been experimentally determined over many years to determine that Si(OH)4 is generally the dominant vapor species.[13] Even more advanced environmental barrier coatings are required to protect these CMCs from water vapor as well as other environmental degradants. For instance, as the gas temperatures increase towards 1400 K-1500 K, sand particles begin to melt and react with coatings. The melted sand is generally a mixture of calcium oxide, magnesium oxide, aluminum oxide, and silicon oxide (commonly referred to as CMAS). Many research groups are investigating the harmful effects of CMAS on turbine coatings and how to prevent damage. CMAS is a large barrier to increasing the combustion temperature of gas turbine engines and will need to be solved before turbines see a large increase in efficiency from temperature increase.[14]
Processing
In industry, thermal barrier coatings are produced in a number of ways:
- Electron beam physical vapor deposition: EBPVD
- Air plasma spray: APS
- High velocity oxygen fuel: HVOF
- Electrostatic spray-assisted vapor deposition: ESAVD
- Direct vapor deposition
Additionally, the development of advanced coatings and processing methods is a field of active research. One such example is the solution precursor plasma spray process, which has been used to create TBCs with some of the lowest reported thermal conductivities without sacrificing thermal cyclic durability.
See also
References
| Wikimedia Commons has media related to Thermal barrier coating. |
- F.Yu and T.D.Bennett (2005). "A nondestructive technique for determining thermal properties of thermal barrier coatings". J. Appl. Phys. 97: 013520. doi:10.1063/1.1826217.
- Clarke, David R.; Phillpot, Simon R. (2005). "Thermal barrier coating materials". Materials Today. 8 (6): 22–29. doi:10.1016/S1369-7021(05)70934-2.
- Cao , Vassen R., Stoever D. (2004). "Ceramic Materials for Thermal Barrier Coatings". Journal of the European Ceramic Society. 24 (1): 1–10. doi:10.1016/s0955-2219(03)00129-8.CS1 maint: multiple names: authors list (link)
- Cao X.Q., Vassen R., Stoever D. (2004). "Ceramic Materials for Thermal Barrier Coatings". Journal of the European Ceramic Society. 24 (1): 1–10. doi:10.1016/s0955-2219(03)00129-8.CS1 maint: multiple names: authors list (link)
- Padture Nitin P.; Gell Maurice; Jordan Eric H. (2002). "Thermal Barrier Coatings for Gas-Turbine Engine Applications". Science. 296 (5566): 280–284. doi:10.1126/science.1068609. PMID 11951028.
- Padture Nitin P.; Gell Maurice; Jordan Eric H. (2002). "Thermal Barrier Coatings for Gas-Turbine Engine Applications". Science. 296 (5566): 280–284. doi:10.1126/science.1068609. PMID 11951028.
- Cao , Vassen R., Stoever D. (2004). "Ceramic Materials for Thermal Barrier Coatings". Journal of the European Ceramic Society. 24 (1): 1–10. doi:10.1016/s0955-2219(03)00129-8.CS1 maint: multiple names: authors list (link)
- Clarke, David R.; Phillpot, Simon R. (2005). "Thermal barrier coating materials". Materials Today. 8 (6): 22–29. doi:10.1016/S1369-7021(05)70934-2.
- Heat engine
- Perepezko J. H. (2009). "The Hotter the Engine, the Better". Science. 326 (5956): 1068–1069. doi:10.1126/science.1179327. PMID 19965415.
- Evans A. G.; Clarke D. R.; Levi C. G. (2008). "The influence of oxides on the performance of advanced gas turbines". Journal of the European Ceramic Society. 28 (7): 1405–1419. doi:10.1016/j.jeurceramsoc.2007.12.023.
- Padture N. P.; Gell M.; Jordan E. H. (2002). "Thermal Barrier Coatings for Gas-Turbine Engine Applications". Science. 296 (5566): 280–284. doi:10.1126/science.1068609. PMID 11951028.
- Jacobson Nathan S.; Opila Elizabeth J.; Myers Dwight L.; Copland Evan H. (2005). "Thermodynamics of gas phase species in the Si–O–H system". The Journal of Chemical Thermodynamics. 37 (10): 1130–1137. doi:10.1016/j.jct.2005.02.001.
- Zhao H.; Levi C. G.; Wadley H. N. G. (2014). "Molten silicate interactions with thermal barrier coatings". Surface and Coatings Technology. 251: 74–86. doi:10.1016/j.surfcoat.2014.04.007.
External links
- "High Temperature Coatings". Wadley research group. University of Virginia.