Trimethylsilane
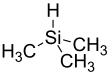
Trimethylsilane is the organosilicon compound with the formula (CH3)3SiH. It is a trialkylsilane. The Si-H bond is reactive. It is less commonly used as a reagent than the related triethylsilane, which is a liquid at room temperature.
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.012.366 | ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| Properties | |||
| C3H10Si | |||
| Molar mass | 74.198 g·mol−1 | ||
| Density | 0.638 g cm−3 | ||
| Melting point | −135.9 °C (−212.6 °F; 137.2 K) | ||
| Boiling point | 6.7 °C (44.1 °F; 279.8 K) | ||
| Hazards | |||
EU classification (DSD) (outdated) |
|||
| R-phrases (outdated) | R12, R36/37/38 | ||
| S-phrases (outdated) | S9, S16, S26, S33 | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trimethylsilane is used in the semi-conductor industry as precursor to deposit dielectrics and barrier layers via plasma-enhanced chemical vapor deposition (PE-CVD).[1] It is also used a source gas to deposit TiSiCN hard coatings via plasma-enhanced magnetron sputtering (PEMS). It has also been used to deposit silicon carbide hard coatings via low-pressure chemical vapor deposition (LP-CVD) at relatively low temperatures <1000 °C. It is an expensive gas but safer to use than silane (SiH4); and produces properties in the coatings that cannot be undertaken by multiple source gases containing silicon and carbon.
See also
- Dimethylsilane
- Trimethylsilyl functional group
References
- Chen, Sheng-Wen; Wang, Yu-Sheng; Hu, Shao-Yu; Lee, Wen-Hsi; Chi, Chieh-Cheng; Wang, Ying-Lang (2012). "A Study of Trimethylsilane (3MS) and Tetramethylsilane (4MS) Based α-SiCN:H/α-SiCO:H Diffusion Barrier Films". Materials. 5 (3): 377. doi:10.3390/ma5030377. PMC 5448926. PMID 28817052.