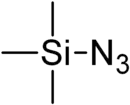Trimethylsilyl azide
Trimethylsilyl azide ((CH3)3SiN3) is a chemical compound used as a reagent in organic chemistry.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Azido(trimethyl)silane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1903730 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.022.798 | ||
| EC Number |
| ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H9N3Si | |||
| Molar mass | 115.211 g·mol−1 | ||
| Appearance | clear liquid, colorless | ||
| Density | 0.8763 g/cm3 (20 °C) | ||
| Melting point | −95 °C (−139 °F; 178 K) | ||
| Boiling point | 52 to 53 °C (126 to 127 °F; 325 to 326 K) at 175 mmHg (92 to 95 °C at 760 mmHg) | ||
| reacts to form dangerous hydrazoic acid | |||
| Hazards | |||
| R-phrases (outdated) | R11, R23, R24, R25, R29, R50, R51, R52, R53 | ||
| S-phrases (outdated) | S16,S29,S36, S37,S45,S57,S8, | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 6 °C (43 °F; 279 K) | ||
| > 300 °C (572 °F; 573 K) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Trimethylsilyl azide is commercially available. It may be prepared by the reaction of trimethylsilyl chloride and sodium azide:[1]
- TMSCl + NaN3 → TMSN3 + NaCl (TMS = (CH3)3Si)
Applications
It is considered a safer replacement for hydrazoic acid in many reactions. It will however over time hydrolyze to hydrazoic acid and therefore it must be stored free of moisture [2] It has been used in the Oseltamivir total synthesis.
Safety
Trimethylsilyl azide is incompatible with moisture, strong oxidizing agents, and strong acids. In 2014, a graduate student at the University of Minnesota was injured in an explosion that occurred in the synthesis of a large batch of trimethylsilyl azide.[3]
References
- L. Birkofer and P. Wegner (1988). "Trimethylsilyl azide". Organic Syntheses.; Collective Volume, 6, p. 1030
- Jafarzadeh, Mohammad (2007). "Trimethylsilyl Azide (TMSN3): A Versatile Reagent in Organic Synthesis". Synlett. 2007 (13): 2144–2145. doi:10.1055/s-2007-984895.
- http://cenblog.org/the-safety-zone/2014/07/more-details-on-the-university-of-minnesota-explosion-and-response/
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.