Tryptophan operon leader
The Tryptophan operon leader is an RNA element found at the 5′ of some bacterial tryptophan operons. The leader sequence can assume two different secondary structures known as the terminator and the anti-terminator structure. The leader also codes for very short peptide sequence that is rich in tryptophan. The terminator structure is recognised as a termination signal for RNA polymerase and the operon is not transcribed. This structure forms when the cell has an excess of tryptophan and ribosome movement over the leader transcript is not impeded.[1] When there is a deficiency of the charged tryptophanyl tRNA the ribosome translating the leader peptide stalls and the antiterminator structure can form. This allows RNA polymerase to transcribe the operon.
| Tryptophan operon leader | |
|---|---|
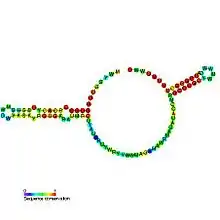 Predicted secondary structure and sequence conservation of Trp_leader | |
| Identifiers | |
| Symbol | Trp_leader |
| Rfam | RF00513 |
| Other data | |
| RNA type | Cis-reg; leader |
| Domain(s) | Bacteria |
| SO | SO:0000233 |
| PDB structures | PDBe |
At least 6 different amino acid operons are known to be regulated by this attenuation.
Trp RNA-binding attenuation protein
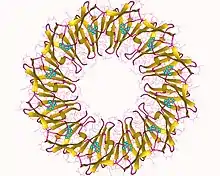
The formation of the terminator requires the trp RNA-binding attenuation protein (TRAP protein) in species of Bacillus and related bacteria. This protein is encoded by the MtrB gene.[2] TRAP protein forms an oligomer of 11 subunits, in the presence of tryptophan this binds to section of RNA containing 11 (G/U)AG repeats. This sequence overlaps the anti-terminator loop, thus TRAP-binding preventing formation of the anti-termintor loop. When TRAP is bound the termintor loop forms and the operon is not transcribed.[3][4]
Tryptophan RNA-binding attenuator protein inhibitory protein
Tryptophan RNA-binding attenuator protein inhibitory protein (Anti-TRAP protein or AT) is a short protein encoded by the rtpA in Bacillus. Synthesis of anti-TRAP is induced by uncharged tryptophan tRNA. Anti-TRAP binds to TRAP and inhibits its binding to the tryptophan operator leader. This causes the anti-terminator loop to form and the tryptophan operon to be transcribed.[3][5][6]
References
- Kolter, R; Yanofsky C (1982). "Attenuation in amino acid biosynthetic operons". Annu Rev Genet. 16: 113–134. doi:10.1146/annurev.ge.16.120182.000553. PMID 6186194.
- Babitzke P, Gollnick P, Yanofsky C (1992). "The mtrAB operon of Bacillus subtilis encodes GTP cyclohydrolase I (MtrA), an enzyme involved in folic acid biosynthesis, and MtrB, a regulator of tryptophan biosynthesis". J Bacteriol. 174 (7): 2059–2064. doi:10.1128/jb.174.7.2059-2064.1992. PMC 205820. PMID 1551827.
- Babitzke P, Gollnick P (2001). "Posttranscription initiation control of tryptophan metabolism in Bacillus subtilis by the trp RNA-binding attenuation protein (TRAP), anti-TRAP, and RNA structure". J Bacteriol. 183 (20): 5795–5802. doi:10.1128/JB.183.20.5795-5802.2001. PMC 99655. PMID 11566976.
- Babitzke P, Schaak J, Yakhnin AV, Bevilacqua PC (2003). "Role of RNA structure in transcription attenuation in Bacillus subtilis: the trpEDCFBA operon as a model system". Methods Enzymol. Methods in Enzymology. 371: 392–404. doi:10.1016/S0076-6879(03)71030-1. ISBN 9780121822743. PMID 14712717.
- Valbuzzi A, Yanofsky C (2001). "Inhibition of the B. subtilis regulatory protein TRAP by the TRAP-inhibitory protein, AT". Science. 293 (5537): 2057–2059. doi:10.1126/science.1062187. PMID 11557884.
- Valbuzzi A, Gollnick P, Babitzke P, Yanofsky C (2002). "The anti-trp RNA-binding attenuation protein (Anti-TRAP), AT, recognizes the tryptophan-activated RNA binding domain of the TRAP regulatory protein". J Biol Chem. 277 (12): 10608–10613. doi:10.1074/jbc.M111813200. PMID 11786553.