UQCR11
UQCR11 (ubiquinol-cytochrome c reductase, complex III sub-unit XI) is a protein that in humans is encoded by the UQCR11 gene.[5][6] UQCR11 is the smallest known component of Complex III in the mitochondrial respiratory chain.[6]
Structure
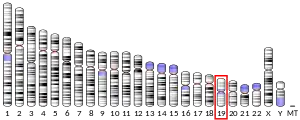
The UQCR11 gene, located on the p arm of chromosome 19 in position 13.3, is made up of 3 exons and is 8,329 base pairs in length.[6] The UQCR11 protein weighs 6.6 kDa and is composed of 56 amino acids.[7][8] This gene encodes the smallest known component of the ubiquinol-cytochrome c reductase complex, which is also known as Complex III and is part of the mitochondrial respiratory chain.[6] In vertebrates, Complex III contains 11 sub-units: 3 respiratory sub-units, 2 core proteins and 6 low-molecular weight proteins.[9][10] Proteobacterial complexes may contain as few as three sub-units.[11]
Function
The UQCR11 protein may function as a binding factor for the iron-sulfur protein in Complex III, which is ubiquitous in human cells.[6] Complex III catalyzes the chemical reaction
- QH2 + 2 ferricytochrome c Q + 2 ferrocytochrome c + 2 H+
Thus, the two substrates of Complex III are dihydroquinone (QH2) and ferri- (Fe3+) cytochrome c, whereas its 3 products are quinone (Q), ferro- (Fe2+) cytochrome c, and H+. This complex belongs to the family of oxidoreductases, specifically those acting on diphenols and related substances as donor with a cytochrome as acceptor. This enzyme participates in oxidative phosphorylation. It has four cofactors: cytochrome c1, cytochrome b-562, cytochrome b-566 and a 2-Iron ferredoxin of the Rieske type.
References
- GRCh38: Ensembl release 89: ENSG00000127540 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000020163 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Eisenberg E, Levanon EY (July 2003). "Human housekeeping genes are compact". Trends in Genetics. 19 (7): 362–365. arXiv:q-bio/0309020. Bibcode:2003q.bio.....9020E. doi:10.1016/S0168-9525(03)00140-9. PMID 12850439. S2CID 7728312.
- "Entrez Gene: UQCR11 ubiquinol-cytochrome c reductase, complex III subunit XI".
- Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (Oct 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- "Cytochrome b-c1 complex subunit 11". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).
- Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, et al. (1998). "Electron transfer by domain movement in cytochrome bc1". Nature. 392 (6677): 677–84. Bibcode:1998Natur.392..677Z. doi:10.1038/33612. PMID 9565029. S2CID 4380033.
- Hao GF, Wang F, Li H, Zhu XL, Yang WC, Huang LS, et al. (2012). "Computational discovery of picomolar Q(o) site inhibitors of cytochrome bc1 complex". J Am Chem Soc. 134 (27): 11168–76. doi:10.1021/ja3001908. PMID 22690928.
- Yang XH, Trumpower BL (1986). "Purification of a three-subunit ubiquinol-cytochrome c oxidoreductase complex from Paracoccus denitrificans". J Biol Chem. 261 (26): 12282–9. PMID 3017970.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.