Ubiquinol
Ubiquinol is an electron-rich (reduced) form of coenzyme Q10.
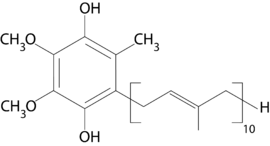 | |
| Names | |
|---|---|
| IUPAC name
2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl]-5,6-dimethoxy-3-methyl-benzene-1,4-diol | |
| Other names
Reduced CoQ10, unoxidized CoQ10, CoQ10H2, or dihydroquinone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | C003741 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C59H92O4 | |
| Molar mass | 865.381 g·mol−1 |
| Appearance | off-white powder |
| Melting point | 45.6 °C (114.1 °F; 318.8 K) |
| practically insoluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side-chain is 9-10 units long in mammals. Coenzyme Q10 (CoQ10) exists in three redox states, fully oxidized (ubiquinone), partially reduced (semiquinone or ubisemiquinone), and fully reduced (ubiquinol). The redox functions of ubiquinol in cellular energy production and antioxidant protection are based on the ability to exchange two electrons in a redox cycle between ubiquinol (reduced) and the ubiquinone (oxidized) form.[1][2]
Bioavailability
It is well-established that CoQ10 is not well absorbed into the body, as has been published in many peer-reviewed scientific journals.[4] Since the ubiquinol form has two additional hydrogens, it results in the conversion of two ketone groups into hydroxyl groups on the active portion of the molecule. This causes an increase in the polarity of the CoQ10 molecule and may be a significant factor behind the observed enhanced bioavailability of ubiquinol.
Content in foods
In foods, there are varying amounts of ubiquinol. An analysis of a range of foods found ubiquinol to be present in 66 out of 70 items and accounted for 46% of the total coenzyme Q10 intake (in the Japanese diet). The following chart is a sample of the results.[5]
| Food | Ubiquinol (μg/g) | Ubiquinone (μg/g) |
|---|---|---|
| Beef (shoulder) | 5.36 | 25 |
| Beef (liver) | 40.1 | 0.4 |
| Pork (shoulder) | 25.4 | 19.6 |
| Pork (thigh) | 2.63 | 11.2 |
| Chicken (breast) | 13.8 | 3.24 |
| Mackerel | 0.52 | 10.1 |
| Tuna (canned) | 14.6 | 0.29 |
| Yellowtail | 20.9 | 12.5 |
| Broccoli | 3.83 | 3.17 |
| Parsley | 5.91 | 1.57 |
| Orange | 0.88 | 0.14 |
Molecular aspects
Ubiquinol is a benzoquinol and is the reduced product of ubiquinone also called coenzyme Q10. Its tail consists of 10 isoprene units.
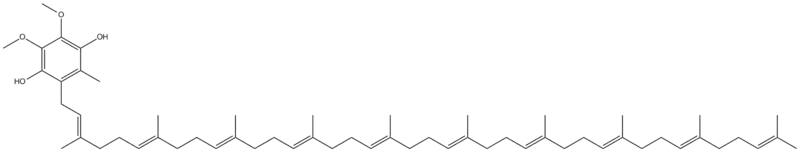
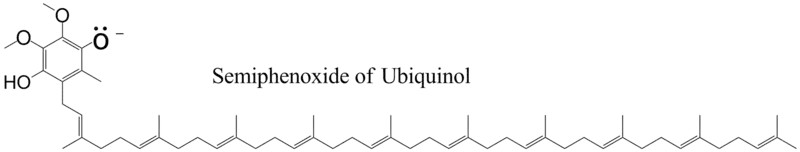
The reduction of ubiquinone to ubiquinol occurs in Complexes I & II in the electron transfer chain. The Q cycle[6] is a process that occurs in cytochrome b,[7][8] a component of Complex III in the electron transport chain, and that converts ubiquinol to ubiquinone in a cyclic fashion. When ubiquinol binds to cytochrome b, the pKa of the phenolic group decreases so that the proton ionizes and the phenoxide anion is formed.
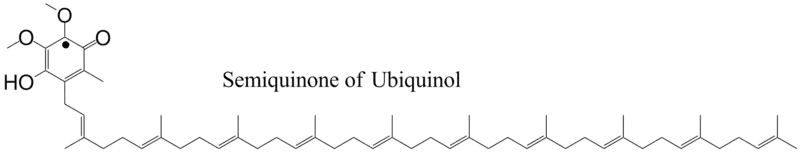
If the phenoxide oxygen is oxidized, the semiquinone is formed with the unpaired electron being located on the ring.
References
- Mellors, A; Tappel, AL (1966). "The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol". The Journal of Biological Chemistry. 241 (19): 4353–6. PMID 5922959.
- Mellors, A.; Tappel, A. L. (1966). "Quinones and quinols as inhibitors of lipid peroxidation". Lipids. 1 (4): 282–4. doi:10.1007/BF02531617. PMID 17805631.
- Banerjee R (2007). Redox Biochemistry. John Wiley & Sons. p. 35. ISBN 978-0-470-17732-7.
- James, Andrew M.; Cochemé, Helena M.; Smith, Robin A. J.; Murphy, Michael P. (2005). "Interactions of Mitochondria-targeted and Untargeted Ubiquinones with the Mitochondrial Respiratory Chain and Reactive Oxygen Species: Implications for the use of exogenous ubiquinones as therapies and experimental tools". Journal of Biological Chemistry. 280 (22): 21295–312. doi:10.1074/jbc.M501527200. PMID 15788391.
- Kubo, Hiroshi; Fujii, Kenji; Kawabe, Taizo; Matsumoto, Shuka; Kishida, Hideyuki; Hosoe, Kazunori (2008). "Food content of ubiquinol-10 and ubiquinone-10 in the Japanese diet". Journal of Food Composition and Analysis. 21 (3): 199–210. doi:10.1016/j.jfca.2007.10.003.
- Slater, E.C. (1983). "The Q cycle, an ubiquitous mechanism of electron transfer". Trends in Biochemical Sciences. 8 (7): 239–42. doi:10.1016/0968-0004(83)90348-1.
- Trumpower BL (June 1990). "Cytochrome bc1 complexes of microorganisms". Microbiol. Rev. 54 (2): 101–29. PMC 372766. PMID 2163487.
- Trumpower, Bernard L. (1990). "The Protonmotive Q Cycle". The Journal of Biological Chemistry. 265 (20): 11409–12. PMID 2164001.