Umeclidinium bromide/vilanterol
Umeclidinium bromide/vilanterol, sold under the brand name Anoro Ellipta, among others, is a fixed-dose combination medication for the treatment of chronic obstructive pulmonary disease (COPD).[3][4] It is applied as an inhalation.
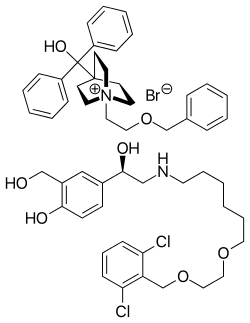 Umeclidinium bromide (TOP), vilanterol (BOTTOM) | |
| Combination of | |
|---|---|
| Umeclidinium bromide | Muscarinic antagonist |
| Vilanterol | Ultra-long-acting β2 agonist |
| Clinical data | |
| Trade names | Anoro Ellipta, Laventair Ellipta |
| AHFS/Drugs.com | Professional Drug Facts |
| License data | |
| Pregnancy category |
|
| Routes of administration | Inhalation (DPI) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| KEGG | |
The most common side effects include upper respiratory tract infections (nose and throat infection), urinary tract infections (infection of the structures that carry urine), pharyngitis (inflammation of the throat), sinusitis (inflammation of the sinuses), nasopharyngitis (inflammation of the nose and throat), headache, cough, oropharyngeal pain (pain in the mouth and throat), constipation and dry mouth.[2]
References
- "Anoro Ellipta 55 micrograms/22 micrograms inhalation powder, pre-dispensed - Summary of Product Characteristics (SmPC)". (emc). 7 October 2019. Retrieved 29 October 2020.
- "Anoro Ellipta EPAR". European Medicines Agency (EMA). Retrieved 28 October 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Feldman GJ, Edin A (December 2013). "The combination of umeclidinium bromide and vilanterol in the management of chronic obstructive pulmonary disease: current evidence and future prospects". Therapeutic Advances in Respiratory Disease. 7 (6): 311–9. doi:10.1177/1753465813499789. PMID 24004659. S2CID 5744282.
- "FDA Approves Umeclidinium and Vilanterol Combo for COPD". Medscape. December 18, 2013.
External links
- "Umeclidinium bromide mixture with vilanterol". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.