Urolithin A
Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria.[1] It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, and walnuts.[2] Since the 2000s, urolithin A has been subject of preliminary studies regarding its possible biological effects.
 | |
| Names | |
|---|---|
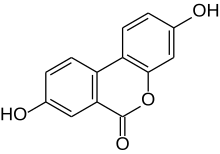
| IUPAC name
3,8-Dihydroxybenzo[c]chromen-6-one | |
| Other names
Uro-A | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H8O4 | |
| Molar mass | 228.203 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Urolithin A is not known to be found in any food source. Its bioavailability mostly depends on individual microbiota composition, as only some bacteria are able to convert ellagitannins into urolithins.[3]
Chemistry
Urolithin A belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. These are polycyclic aromatic compounds containing a 1-benzopyran moiety with a ketone group at the C2 carbon atom (1-benzopyran-2-one).
Biochemistry and metabolism
Pomegranate fruits, walnuts or raspberries are sources of ellagitannins.[4][5][6] Ellagitannins are hydrolyzed in the gut to release ellagic acid, which is further processed by the gut microflora into urolithins through the loss of one of its two lactones and by successive removal of hydroxyl groups.[7]
While studies have shown that Gordonibacter urolithinfaciens and Gordonibacter pamelaeae play a role in the conversion of ellagic acids and ellagitannins into urolithin A, the microorganisms responsible for the complete transformation into the final urolithins are still unknown.[3] The efficiency of the conversion of ellagitannins into urolithin A significantly varies in humans, and some individuals do not show any conversion.[8]
When synthesized and absorbed in the intestines, urolithin A enters the systemic circulation where it becomes available to tissues throughout the body where it is further subjected to additional chemical transformations (including glucuronidation, methylation, sulfation, or a combination of them) within the enterocytes and hepatocytes.[9] Urolithin A and its derivatives - urolithin A glucuronide and urolithin A sulfate being most abundant - release into the circulation,[10][11] before being excreted in the urine.[12][13]
Potential mechanism of action
In laboratory studies, urolithin A was shown to induce mitophagy, which is a selective recycling of mitochondria by autophagy, a process that cleans defective mitochondria following damage or stress, and tends to become less efficient during aging.[14] This effect has been observed in different animal species (mammalian cells, rodents and C. elegans).[14]
Safety
In vivo studies did not determine any toxicity or specific adverse effects following dietary intake of urolithin A.[15] Safety studies in elderly humans indicated urolithin A was well tolerated.[16] In 2018, the US Food and Drug Administration listed urolithin A as a safe ingredient for food or dietary supplement products having content in the range of 250 mg to one gram per serving.[17]
Dietary sources
Urolithin A is not known to be found in any food. It forms as the result of transformation of ellagic acids and ellagitannins by the gut microflora in humans. Ellagic acid itself results from the hydrolysis of ellagitannins in the gut in the presence of water.
Sources of ellagitannins are: pomegranates, nuts, some berries (raspberries, strawberries, blackberries, cloudberries), tea, muscadine grapes, many tropical fruits, and oak-aged wines (table below).
The conversion of the ellagic acids into urolithin A depends on individual microflora composition and can vary significantly.[8][18]
| Dietary source | Ellagic Acid[19] |
|---|---|
| Fruits (mg/100g fresh weight) | |
| Blackberries | 150 |
| Black raspberries | 90 |
| Boysenberries | 70 |
| Cloudberries | 315.1 |
| Pomegranate | 269.9[20] |
| Raspberries | 270 |
| Rose hip | 109.6 |
| Strawberries | 77.6 |
| Strawberry jam | 24.5 |
| Yellow raspberries | 1900 |
| Nuts (mg/g) | |
| Pecans | 33 |
| Walnuts | 59 |
| Beverages (mg/L) | |
| Pomegranate juice | 811.1[20] |
| Cognac | 31-55 |
| Oak-aged red wine | 33 |
| Whiskey | 1.2 |
| Seeds (mg/g) | |
| Black raspberries | 6.7 |
| Red raspberries | 8.7 |
| Boysenberries | 30 |
| Mango | 1.2 |
Research
Laboratory research on the potential biological roles of urolithin A includes studies of lifespan and muscle function.[14][16]
See also
References
- Garcia-Muñoz, Cristina; Vaillant, Fabrice (2014-12-02). "Metabolic Fate of Ellagitannins: Implications for Health, and Research Perspectives for Innovative Functional Foods". Critical Reviews in Food Science and Nutrition. 54 (12): 1584–1598. doi:10.1080/10408398.2011.644643. ISSN 1040-8398. PMID 24580560. S2CID 5387712.
- Cerdá, Begoña; Tomás-Barberán, Francisco A.; Espín, Juan Carlos (2005-01-01). "Metabolism of Antioxidant and Chemopreventive Ellagitannins from Strawberries, Raspberries, Walnuts, and Oak-Aged Wine in Humans: Identification of Biomarkers and Individual Variability". Journal of Agricultural and Food Chemistry. 53 (2): 227–235. doi:10.1021/jf049144d. ISSN 0021-8561. PMID 15656654.
- Selma, María V.; Beltrán, David; Luna, María C.; Romo-Vaquero, María; García-Villalba, Rocío; Mira, Alex; Espín, Juan C.; Tomás-Barberán, Francisco A. (2017). "Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid". Frontiers in Microbiology. 8: 1521. doi:10.3389/fmicb.2017.01521. ISSN 1664-302X. PMC 5545574. PMID 28824607.
- Johanningsmeier, Suzanne D.; Harris, G. Keith (2011-02-28). "Pomegranate as a Functional Food and Nutraceutical Source". Annual Review of Food Science and Technology. 2 (1): 181–201. doi:10.1146/annurev-food-030810-153709. ISSN 1941-1413. PMID 22129380.
- Sánchez-González, Claudia; Ciudad, Carlos J.; Noé, Véronique; Izquierdo-Pulido, Maria (2017-11-02). "Health benefits of walnut polyphenols: An exploration beyond their lipid profile". Critical Reviews in Food Science and Nutrition. 57 (16): 3373–3383. doi:10.1080/10408398.2015.1126218. hdl:2445/99551. ISSN 1040-8398. PMID 26713565. S2CID 19611576.
- Ludwig, Iziar A.; Mena, Pedro; Calani, Luca; Borges, Gina; Pereira-Caro, Gema; Bresciani, Letizia; Rio, Daniele Del; Lean, Michael E.J.; Crozier, Alan (2015). "New insights into the bioavailability of red raspberry anthocyanins and ellagitannins" (PDF). Free Radical Biology and Medicine. 89: 758–769. doi:10.1016/j.freeradbiomed.2015.10.400. PMID 26475039.
- Espín, Juan Carlos; Larrosa, Mar; García-Conesa, María Teresa; Tomás-Barberán, Francisco (2013). "Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far". Evidence-Based Complementary and Alternative Medicine. 2013: 270418. doi:10.1155/2013/270418. ISSN 1741-427X. PMC 3679724. PMID 23781257.
- Tomás-Barberán, Francisco A.; González-Sarrías, Antonio; García-Villalba, Rocío; Núñez-Sánchez, María A.; Selma, María V.; García-Conesa, María T.; Espín, Juan Carlos (2017-01-01). "Urolithins, the rescue of "old" metabolites to understand a "new" concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status". Molecular Nutrition & Food Research. 61 (1): n/a. doi:10.1002/mnfr.201500901. ISSN 1613-4133. PMID 27158799.
- Tulipani, Sara; Urpi-Sarda, Mireia; Garcı́a-Villalba, Rocío; Rabassa, Montserrat; López-Uriarte, Patricia; Bulló, Mònica; Jáuregui, Olga; Tomás-Barberán, Francisco; Salas-Salvadó, Jordi (2012-09-12). "Urolithins Are the Main Urinary Microbial-Derived Phenolic Metabolites Discriminating a Moderate Consumption of Nuts in Free-Living Subjects with Diagnosed Metabolic Syndrome". Journal of Agricultural and Food Chemistry. 60 (36): 8930–8940. doi:10.1021/jf301509w. hdl:2445/171748. ISSN 0021-8561. PMID 22631214.
- Seeram, Navindra P.; Zhang, Yanjun; McKeever, Rodney; Henning, Susanne M.; Lee, Ru-po; Suchard, Marc A.; Li, Zhaoping; Chen, Steve; Thames, Gail (2008-06-01). "Pomegranate Juice and Extracts Provide Similar Levels of Plasma and Urinary Ellagitannin Metabolites in Human Subjects". Journal of Medicinal Food. 11 (2): 390–394. doi:10.1089/jmf.2007.650. ISSN 1096-620X. PMC 3196216. PMID 18598186.
- Mertens-Talcott, Susanne U.; Jilma-Stohlawetz, Petra; Rios, Jolian; Hingorani, Lal; Derendorf, Hartmut (2006-11-01). "Absorption, Metabolism, and Antioxidant Effects of Pomegranate (Punica granatum L.) Polyphenols after Ingestion of a Standardized Extract in Healthy Human Volunteers". Journal of Agricultural and Food Chemistry. 54 (23): 8956–8961. doi:10.1021/jf061674h. ISSN 0021-8561. PMID 17090147.
- González-Sarrías, Antonio; Giménez-Bastida, Juan A.; García-Conesa, María T.; Gómez-Sánchez, María B.; García-Talavera, Noelia V.; Gil-Izquierdo, Angel; Sánchez-Álvarez, Carmen; Fontana-Compiano, Luis O.; Morga-Egea, Juan P. (2010-03-01). "Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice". Molecular Nutrition & Food Research. 54 (3): 311–322. doi:10.1002/mnfr.200900152. ISSN 1613-4133. PMID 19885850.
- Truchado, Pilar; Larrosa, Mar; García-Conesa, María Teresa; Cerdá, Begoña; Vidal-Guevara, María Luisa; Tomás-Barberán, Francisco A.; Espín, Juan Carlos (2012-06-13). "Strawberry Processing Does Not Affect the Production and Urinary Excretion of Urolithins, Ellagic Acid Metabolites, in Humans". Journal of Agricultural and Food Chemistry. 60 (23): 5749–5754. doi:10.1021/jf203641r. ISSN 0021-8561. PMID 22126674.
- Ryu, Dongryeol; Mouchiroud, Laurent; Andreux, Pénélope A; Katsyuba, Elena; Moullan, Norman; Nicolet-dit-Félix, Amandine A; Williams, Evan G; Jha, Pooja; Sasso, Giuseppe Lo (2016). "Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents". Nature Medicine. 22 (8): 879–888. doi:10.1038/nm.4132. PMID 27400265. S2CID 9789056.
- Heilman, Jacqueline; Andreux, Pénélope; Tran, Nga; Rinsch, Chris; Blanco-Bose, William (2017). "Safety assessment of urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid". Food and Chemical Toxicology. 108 (Pt A): 289–297. doi:10.1016/j.fct.2017.07.050. PMID 28757461.
- Singh, A.; Andreux, P.; Blanco-Bose, W.; Ryu, D.; Aebischer, P.; Auwerx, J.; Rinsch, C. (2017-07-01). "Orally administered urolithin A is safe and modulates muscle and mitochondrial biomarkers in elderly". Innovation in Aging. 1 (suppl_1): 1223–1224. doi:10.1093/geroni/igx004.4446. PMC 6183836.
- "FDA GRAS notice GRN No. 791: urolithin A". US Food and Drug Administration. 20 December 2018. Retrieved 25 August 2020.
- Selma, María V.; Romo-Vaquero, María; García-Villalba, Rocío; González-Sarrías, Antonio; Tomás-Barberán, Francisco A.; Espín, Juan C. (2016-04-20). "The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism". Food Funct. 7 (4): 1769–1774. doi:10.1039/c5fo01100k. ISSN 2042-650X. PMID 26597167.
- Landete, J.M. (2011). "Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health". Food Research International. 44 (5): 1150–1160. doi:10.1016/j.foodres.2011.04.027.
- García-Villalba, Rocío; Espín, Juan Carlos; Tomás-Barberán, Francisco A. (2016). "Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid". Journal of Chromatography A. 1428: 162–175. doi:10.1016/j.chroma.2015.08.044. PMID 26341594.
External links
- Urolithin A at Phenol-Explorer.eu
- Urolithin A at the Human Metabolome Database