Vinyl sulfone
In organic chemistry, a vinyl sulfone is a functional group consisting of a vinyl group bonded to a sulfone group. Specific compounds containing this functional group are divinyl sulfone,[1] phenyl vinyl sulfone,[2] methyl vinyl sulfone,[3] and ethyl vinyl sulfone.[4]
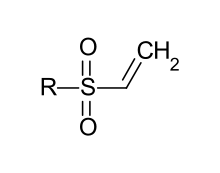
The sulfone makes the vinyl group electrophilic, allowing it to be used as a pharmacophore for binding to the thiol of threonine residues.[5] This same reactive nature is responsible for their major industrial use in vinyl sulfone dyes.[6]
Uses
Vinyl sulfone has uses as a molluscicide pesticide.[7]
Phenyl vinyl sulfone has been applied to ruthenium chemistry as part of olefin metathesis reactions.[8]
Vinyl sulfone has applications to protein purification, especially when linked with mercaptoethanol.[9]
References
- Leonard, Edward C. (1970). Vinyl and Diene Monomers, Part 3. Wiley-Interscience. p. 1466.
- Gustavson, Clarence (1952). Reactions of Phenyl Vinyl Sulfone with Organometallic Reagents. Syracuse University.
- Keith, Lawrence H.; Walters, Douglas B. (1991). The National Toxicology Program's Chemical Data Compendium (Volume 8 ed.). CRC Press. ISBN 9780873717229.
- Leonard, Edward C. (1970). Vinyl and Diene Monomers, Part 3. Wiley-Interscience. p. 1475.
- Patterson, Cam; Cyr, Douglas M., eds. (2005). Ubiquitin-Proteasome Protocols. Springer Science & Business Media. p. PA7. ISBN 9781592598953.
- Research In Technology Of Synthetic Dyes, Pigments And Intermediates. Engineers India. 2005. ISBN 9788186732519.
- Pesticides Abstracts. U.S. Environmental Protection Agency, Office of Pesticide Programs, Program Support Division. 1975.
- Bruneau, Christian; Dixneuf, Pierre H. (2004). Ruthenium Catalysts and Fine Chemistry. Springer Science & Business Media. ISBN 9783540205432.
- Scopes, Robert K. (1993). Protein Purification: Principles and Practice. Springer Science & Business Media. p. 184. ISBN 9780387940724.