Wagner–Meerwein rearrangement
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.[1][2] They can be described as cationic [1,2]-sigmatropic rearrangements, proceeding suprafacially and with stereochemical retention. As such, a Wagner–Meerwein shift is a thermally allowed pericyclic process with the Woodward-Hoffmann symbol [ω0s + σ2s]. They are usually facile, and in many cases, they can take place at temperatures as low as –120 °C. The reaction is named after the Russian chemist Yegor Yegorovich Vagner; he had German origin and published in German journals as Georg Wagner; and Hans Meerwein.
Several reviews have been published.[3][4][5][6][7]
The rearrangement was first discovered in bicyclic terpenes for example the conversion of isoborneol to camphene:[8]

The story of the rearrangement reveals that many scientists were puzzled with this and related reactions and its close relationship to the discovery of carbocations as intermediates.[9]
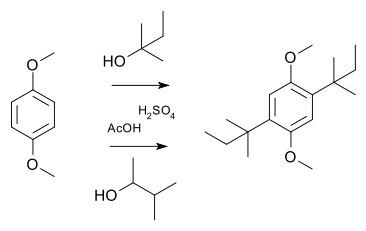
In a simple demonstration reaction of 1,4-dimethoxybenzene with either 2-methyl-2-butanol or 3-methyl-2-butanol in sulfuric acid and acetic acid yields the same disubstituted product,[10] the latter via a hydride shift of the cationic intermediate:
Currently, there are works relating to the use of skeletal rearrangement in the synthesis of bridged azaheterocycles. These data are summarized in [11]
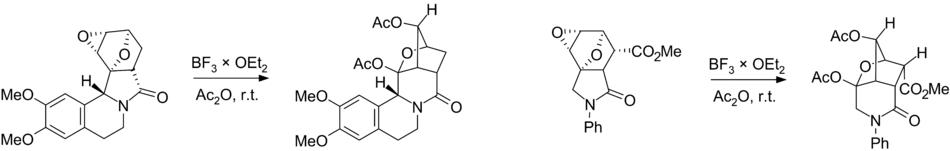
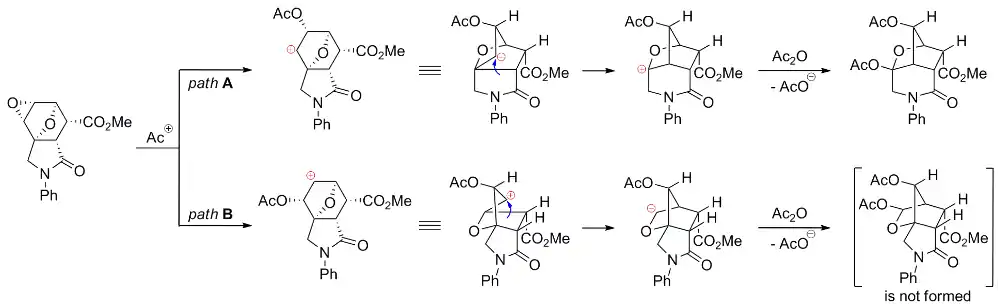
Plausible mechanisms of the Wagner–Meerwein rearrangement of diepoxyisoindoles:
The related Nametkin rearrangement named after Sergey Namyotkin involves the rearrangement of methyl groups in certain terpenes. In some cases the reaction type is also called a retropinacol rearrangement (see Pinacol rearrangement).
References
- Wagner, G. (1899). J. Russ. Phys. Chem. Soc. 31: 690. Missing or empty
|title=(help) - Hans Meerwein (1914). "Über den Reaktionsmechanismus der Umwandlung von Borneol in Camphen; [Dritte Mitteilung über Pinakolinumlagerungen.]". Justus Liebig's Annalen der Chemie. 405 (2): 129–175. doi:10.1002/jlac.19144050202.
- Popp, F. D.; McEwen, W. E. (1958). "Polyphosphoric Acids As a Reagent in Organic Chemistry". Chem. Rev. 58 (2): 375. doi:10.1021/cr50020a004.
- Cargill, Robert L.; Jackson, Thomas E.; Peet, Norton P.; Pond, David M. (1974). "Acid-catalyzed rearrangements of β,γ-unsaturated ketones". Acc. Chem. Res. 7 (4): 106–113. doi:10.1021/ar50076a002.
- Olah, G. A. (1976). "Stable carbocations, 189. The σ-bridged 2-norbornyl cation and its significance to chemistry". Acc. Chem. Res. 9 (2): 41–52. doi:10.1021/ar50098a001.
- Hogeveen, H.; Van Krutchten, E. M. G. A. (1979). "Wagner-meerwein rearrangements in long-lived polymethyl substituted bicyclo[3.2.0]heptadienyl cations". Top. Curr. Chem. Topics in Current Chemistry. 80: 89–124. doi:10.1007/BFb0050203. ISBN 3-540-09309-5.CS1 maint: multiple names: authors list (link)
- Hanson, J. R. (1991). "Wagner–Meerwein Rearrangements". Compr. Org. Synth. 3: 705–719. doi:10.1016/B978-0-08-052349-1.00077-9. ISBN 978-0-08-052349-1.
- March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- Birladeanu, L. (2000). "The Story of the Wagner-Meerwein Rearrangement". J. Chem. Educ. 77 (7): 858–863. Bibcode:2000JChEd..77..858B. doi:10.1021/ed077p858.
- Polito, Victoria; Hamann, Christian S.; Rhile, Ian J. (2010). "Carbocation Rearrangement in an Electrophilic Aromatic Substitution Discovery Laboratory". Journal of Chemical Education. 87 (9): 969. Bibcode:2010JChEd..87..969P. doi:10.1021/ed9000238.
- Zubkov, F. I.; Zaytsev, V. P.; Nikitina, E. V.; Khrustalev, V. N.; Gozun, S. V.; Boltukhina, E. V.; Varlamov, A. V. (2011). "Skeletal Wagner–Meerwein rearrangement of perhydro-3a,6;4,5-diepoxyisoindoles". Tetrahedron. 67 (47): 9148. doi:10.1016/j.tet.2011.09.099.