Wood–Ljungdahl pathway
The Wood–Ljungdahl pathway is a set of biochemical reactions used by some bacteria and archaea called acetogens and methanogens, respectively. It is also known as the reductive acetyl-coenzyme A (Acetyl-CoA) pathway.[1] This pathway enables these organisms to use hydrogen as an electron donor, and carbon dioxide as an electron acceptor and as a building block for biosynthesis.
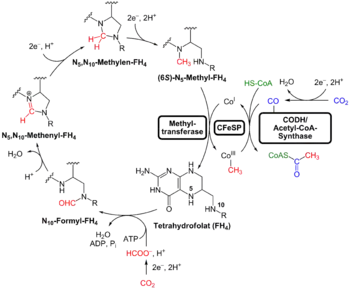
In this pathway carbon dioxide is reduced to carbon monoxide and formic acid or directly into a formyl group, the formyl group is reduced to a methyl group and then combined with the carbon monoxide and Coenzyme A to produce acetyl-CoA. Two specific enzymes participate on the carbon monoxide side of the pathway: CO Dehydrogenase and acetyl-CoA synthase. The former catalyzes the reduction of the CO2 and the latter combines the resulting CO with a methyl group to give acetyl-CoA.[1][2]
Some anaerobic bacteria and archaea use the Wood–Ljungdahl pathway in reverse to break down acetate. For example, some methanogens break down acetate to a methyl group and carbon monoxide, and then reduce the methyl group to methane while oxidizing the carbon monoxide to carbon dioxide.[3] Sulfate reducing bacteria, meanwhile, oxidize acetate completely to CO2 and H2 coupled with the reduction of sulfate to sulfide.[4] When operating in the reverse direction, the acetyl-CoA synthase is sometimes called acetyl-CoA decarbonylase.
The pathway occurs in both bacteria (e.g. acetogens) and archaea (e.g. methanogens[5]). Unlike the Reverse Krebs cycle and the Calvin cycle, this process is not cyclic. A recent study of the genomes of a set of bacteria and archaea suggests that the last universal common ancestor (LUCA) of all cells was using the Wood–Ljungdahl pathway in a hydrothermal setting.[6] Phylometabolic reconstructions also supports this.[7] However, recent experiments have tried to replicate this pathway by attempting to reduce CO2, with very little pyruvate observed using native iron as a reducing agent (<0.03 mM),[8] and even less so under hydrothermal settings with H2 (10 μM).[9]
References
- Ragsdale Stephen W (2006). "Metals and Their Scaffolds To Promote Difficult Enzymatic Reactions". Chem. Rev. 106 (8): 3317–3337. doi:10.1021/cr0503153. PMID 16895330.
- Paul A. Lindahl "Nickel-Carbon Bonds in Acetyl-Coenzyme A Synthases/Carbon Monoxide Dehydrogenases" Met. Ions Life Sci. 2009, volume 6, pp. 133–150. doi:10.1039/9781847559159-00133
- Can, Mehmet; Armstrong, Fraser A.; Ragsdale, Stephen W. (2014-04-23). "Structure, Function, and Mechanism of the Nickel Metalloenzymes, CO Dehydrogenase, and Acetyl-CoA Synthase". Chemical Reviews. 114 (8): 4149–4174. doi:10.1021/cr400461p. ISSN 0009-2665. PMC 4002135. PMID 24521136.
- Spormann, Alfred M.; Thauer, Rudolf K. (1988). "Anaerobic acetate oxidation to CO2 by Desulfotomaculum acetoxidans". Archives of Microbiology. 150 (4): 374–380. doi:10.1007/BF00408310. ISSN 0302-8933.
- Matschiavelli, N.; Oelgeschlager, E.; Cocchiararo, B.; Finke, J.; Rother, M. (2012). "Function and regulation of isoforms of carbon monoxide dehydrogenase/acetyl-CoA synthase in Methanosarcina acetivorans". Journal of Bacteriology. 194 (19): 5377–87. doi:10.1128/JB.00881-12. PMC 3457241. PMID 22865842.
- M. C. Weiss; et al. (2016). "The physiology and habitat of the last universal common ancestor". Nature Microbiology. 1 (16116): 16116. doi:10.1038/nmicrobiol.2016.116. PMID 27562259.
- Braakman, Rogier; Smith, Eric (2012-04-19). "The Emergence and Early Evolution of Biological Carbon-Fixation". PLOS Computational Biology. 8 (4): e1002455. Bibcode:2012PLSCB...8E2455B. doi:10.1371/journal.pcbi.1002455. ISSN 1553-7358. PMC 3334880. PMID 22536150.
- Varma, Sreejith J.; Muchowska, Kamila B.; Chatelain, Paul; Moran, Joseph (2018-04-23). "Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway". Nature Ecology & Evolution. 2 (6): 1019–1024. doi:10.1038/s41559-018-0542-2. ISSN 2397-334X. PMC 5969571. PMID 29686234.
- Preiner, Martina; Igarashi, Kensuke; Muchowska, Kamila B.; Yu, Mingquan; Varma, Sreejith J.; Kleinermanns, Karl; Nobu, Masaru K.; Kamagata, Yoichi; Tüysüz, Harun; Moran, Joseph; Martin, William F. (April 2020). "A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism". Nature Ecology & Evolution. 4 (4): 534–542. doi:10.1038/s41559-020-1125-6. ISSN 2397-334X.
Other reading
- Wood HG (February 1991). "Life with CO or CO2 and H2 as a source of carbon and energy". FASEB J. 5 (2): 156–63. doi:10.1096/fasebj.5.2.1900793. PMID 1900793.
- Diekert G, Wohlfarth G (1994). "Metabolism of homoacetogens". Antonie van Leeuwenhoek. 66 (1–3): 209–21. doi:10.1007/BF00871640. PMID 7747932.