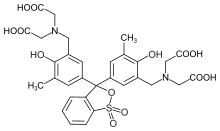
Xylenol orange
Xylenol orange is an organic reagent, most commonly used as a tetrasodium salt as an indicator for metal titrations. When used for metal titrations, it will appear red in the titrand and become yellow once it reaches its endpoint. Historically, commercial preparations of it have been notoriously impure,[1] sometimes consisting of as little as 20% xylenol orange, and containing large amounts of semi-xylenol orange and iminodiacetic acid. Purities as high as 90% are now available.
 | |
| Names | |
|---|---|
| IUPAC name
3,3′-Bis[N,N-bis(carboxymethyl)aminomethyl]- | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.049 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C31H32N2O13S | |
| Molar mass | 672.66 g·mol−1 |
| Melting point | 195 °C (383 °F; 468 K) |
| 200 mg/mL | |
| Hazards | |
| R-phrases (outdated) | R10, R20, R21, R22, R36, R38 |
| S-phrases (outdated) | (S1/2), S26, S27, S28, S62, S63 |
| NFPA 704 (fire diamond) | |
| Flash point | > 93 °C (199 °F; 366 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is fluorescent, and has excitation maximums of 440 & 570 nm and an emission maximum of 610 nm.[2]
References
- Gay, Craig; Collins, James; Gebicki, Janusz M. (1999), "Determination of Iron in Solutions with the Ferric–Xylenol Orange Complex", Analytical Biochemistry, 273 (2): 143–148, doi:10.1006/abio.1999.4207, PMID 10469483
- "Fluorescence - Fluorochrome Data Tables". Molecular Expressions Microscopy Primer: Specialized Microscopy Techniques. 2018-09-11. Retrieved 2020-01-30.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
