YcaO
YcaO is a protein found in bacteria which is involved in the synthesis of thiazole/oxazole modified microcin antibiotics, such as bottromycin. YcaO performs ATP dependent cylodehydration to form the oxazole and thiazole moieties of the microcin.[2][3][4]
| YcaO-like family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
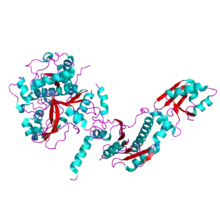 Structure of the heterocyclase TruD. A YcaO-like protein from Prochloron sp. 06037A PDB entry 4bs9[1] | |||||||||
| Identifiers | |||||||||
| Symbol | YcaO | ||||||||
| Pfam | PF02624 | ||||||||
| InterPro | IPR003776 | ||||||||
| |||||||||
References
- Koehnke, J.; Bent, A. F.; Zollman, D.; Smith, K.; Houssen, W. E.; Zhu, X.; Mann, G.; Lebl, T.; Scharff, R.; Shirran, S.; Botting, C. H.; Jaspars, M.; Schwarz-Linek, U.; Naismith, J. H. (2013). "The Cyanobactin Heterocyclase Enzyme: A Processive Adenylase That Operates with a Defined Order of Reaction". Angewandte Chemie International Edition. 52 (52): 13991–13996. doi:10.1002/anie.201306302. PMC 3995012. PMID 24214017.
- Dunbar, K. L.; Melby, J. O.; Mitchell, D. A. (2012). "YcaO domains use ATP to activate amide backbones during peptide cyclodehydrations". Nature Chemical Biology. 8 (6): 569–575. doi:10.1038/nchembio.944. PMC 3428213. PMID 22522320.
- Dunbar KL, Chekan JR, Cox CL, Burkhart BJ, Nair SK, Mitchell DA (2014). "Discovery of a new ATP-binding motif involved in peptidic azoline biosynthesis". Nature Chemical Biology. 10 (10): 823–9. doi:10.1038/nchembio.1608. PMC 4167974. PMID 25129028.
- Koehnke J, Mann G, Bent AF, Ludewig H, Shirran S, Botting C, Lebl T, Houssen WE, Jaspars M, Naismith JH (2015). "Structural analysis of leader peptide binding enables leader-free cyanobactin processing". Nature Chemical Biology. 11 (8): 558–63. doi:10.1038/nchembio.1841. PMC 4512242. PMID 26098679.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.