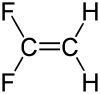
1,1-Difluoroethylene
1,1-Difluoroethylene, also known as vinylidene fluoride, is a hydrofluoroolefin. It is a flammable gas. Global production in 1999 was approximately 33,000 metric tons.[3] It is primarily used in the production of fluoropolymers such as polyvinylidene fluoride.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1-Difluoroethene | |||
| Other names
Difluoro-1,1-ethylene; R-1132a; Halocarbon 1132 A; Freon 1132A; Vinylidene difluoride; Vinylidene fluoride[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | VDF | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.789 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1959 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H2F2 | |||
| Molar mass | 64.035 g·mol−1 | ||
| Appearance | Colorless gas[2] | ||
| Odor | Slightly ethereal[1] | ||
| Density | 2.89 kg/m3 (vapor, 0 °C)[2] 1.122 g/mL (liquid, -84 °C)[2] | ||
| Melting point | −144 °C (−227 °F; 129 K)[2] | ||
| Boiling point | −84 °C (−119 °F; 189 K)[2] | ||
| 0.254 g/L[3] | |||
| Vapor pressure | 35.2 atm (20°C)[4] | ||
| Hazards | |||
| Main hazards | Flammable[4] | ||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |||
| 380 °C (716 °F; 653 K)[1] | |||
| Explosive limits | 5.5%-21.3%[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[4] | ||
REL (Recommended) |
TWA 1 ppm C 5 ppm[4] | ||
IDLH (Immediate danger) |
N.D.[4] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
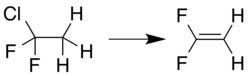
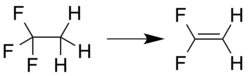
Preparation
1,1-Difluoroethylene can be prepared by elimination reaction from a 1,1,1-trihaloethane compound, for example, loss of hydrogen chloride from 1-chloro-1,1-difluoroethane:.[5]
or loss of hydrogen fluoride from 1,1,1-trifluoroethane:[6]
See also
References
- "Difluoro-1,1-ethylene". Gas Encyclopaedia. Air Liquide.
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- "1,1'-Difluoroethylene (VDF,VF2)" (PDF). International Programme on Chemical Safety.
- NIOSH Pocket Guide to Chemical Hazards. "#0662". National Institute for Occupational Safety and Health (NIOSH).
- Kirk-Othmer Encyclopedia of Chemical Technology (4 ed.). John Wiley and Sons. 1994. pp. V11. Retrieved 8 July 2017.
- Gerhartz, W (1985). Ullmann's Encyclopedia of Industrial Chemistry (5 ed.). VCH Publisher. pp. VA11. Retrieved 8 July 2017.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.