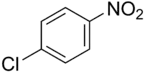
4-Nitrochlorobenzene
4-Nitrochlorobenzene is the organic compound with the formula ClC6H4NO2. It is a pale yellow solid. 4-Nitrochlorobenzene is a common intermediate in the production of a number of industrially useful compounds, including common antioxidants found in rubber. Other isomers with the formula ClC6H4NO2 include 2-nitrochlorobenzene and 3-nitrochlorobenzene.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Chloro-4-nitrobenzene | |||
| Other names
4-Chloro-1-nitrobenzene 4-Chloronitrobenzene p-Nitrochlorobenzene PNCBO | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.002.554 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H4ClNO2 | |||
| Molar mass | 157.55 g·mol−1 | ||
| Appearance | Light yellow solid | ||
| Odor | sweet[1] | ||
| Density | 1.52 g/cm3 (20 °C) | ||
| Melting point | 83.6 °C (182.5 °F; 356.8 K) | ||
| Boiling point | 242.0 °C (467.6 °F; 515.1 K) | ||
| Insoluble | |||
| Solubility in other solvents | Soluble in toluene, ether, acetone, hot ethanol | ||
| Vapor pressure | 0.2 mmHg (30°C)[1] | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| Flash point | 12 °C (54 °F; 285 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
812 mg/kg (rat, oral) 1414 mg/kg (mouse, oral) 440 mg/kg (mouse, oral) 420 mg/kg (rat, oral)[1] | ||
LC50 (median concentration) |
164 mg/m3 (cat, 7 hr)[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 1 mg/m3 [skin][1] | ||
REL (Recommended) |
Ca[1] | ||
IDLH (Immediate danger) |
Ca [100 mg/m3][1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
4-Nitrochlorobenzene is prepared industrially by nitration of chlorobenzene:[2]
This reaction affords both the 2- and the 4-nitro derivatives, in about a 1:2 ratio. These isomers are separated by distillation. 4-Nitrochlorobenzene was originally prepared by the nitration of 4-bromochlorobenzene by Holleman and coworkers.[3]
Applications
4-Nitrochlorobenzene is an intermediate in the preparation of a variety of derivatives. Nitration gives 2,4-dinitrochlorobenzene, and 3,4-dichloronitrobenzene. Reduction with iron metal gives 4-chloroaniline. The electron-withdrawing nature of the appended nitro-group makes the benzene ring especially susceptible to nucleophilic aromatic substitution, unlike related chlorobenzene. Thus, the strong nucleophiles hydroxide, methoxide, and amide displace chloride to give respectively 4-nitrophenol, 4-nitroanisole, 4-nitroaniline.[2]
Another major use of 4-nitrochlorobenzene is its condensation with aniline to produce 4-nitrodiphenylamine. Reductive alkylation of the nitro group affords secondary aryl amines, which are useful antioxidants for rubber.
Safety
The National Institute for Occupational Safety and Health considers 4-nitrochlorobenzene as a potential occupational carcinogen that may be absorbed through the skin.[4] The Occupational Safety and Health Administration set a permissible exposure limit of 1 mg/m3, while the American Conference of Governmental Industrial Hygienists recommends an airborne exposure limit of 0.64 mg/m3, over a time-weighted average of eight hours.[5][6]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0452". National Institute for Occupational Safety and Health (NIOSH).
- Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- "The nitration of mixed dihalogen benzenes" Recueil des Travaux Chimiques des Pays-Bas et de la Belgique. Amsterdam, 1915; pp. 204-235.
- CDC - Immediately Dangerous to Life or Health Concentrations (IDLH): p-nitrochlorobenzene
- CDC - NIOSH Pocket Guide to Chemical Hazards
- New Jersey Department of Health and Senior Services - Hazardous Substance Fact Sheet