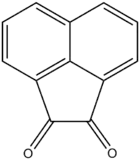
Acenaphthoquinone
Acenaphthoquinone is a quinone derived from acenaphthene. It is a water-insoluble yellow solid. It is a precursor to some agrichemicals and dyes.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acenaphthylene-1,2-dione | |
| Other names
Acenaphthoquinone (no longer accepted even in general nomenclature[1]) Acenaphthenequinone 1,2-Acenaphthenequinone Acenaphthenedione 1,2-Acenaphthylenedione Acenaphthene-1,2-dione 1,2-Diketoacenaphthene | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.311 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H6O2 | |
| Molar mass | 182.178 g·mol−1 |
| Appearance | Purple-yellow crystals to brown powder |
| Melting point | 257 to 261 °C (495 to 502 °F; 530 to 534 K) |
| Insoluble (90.1 mg/l) | |
| Hazards | |
| Main hazards | Irritating |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
The compound is prepared in the laboratory by oxidation of acenaphthene with potassium dichromate.[3] Commercially, oxidation is effected with peroxide. Over-oxidation gives naphthalenedicarboxylic anhydride.[2]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 724. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke (2000). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227.
- Allen, C. F. H.; VanAllan, J. A. (1944). "Acenaphthenequinone". Org. Synth. 24: 1. doi:10.15227/orgsyn.024.0001.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
