Adenoviral keratoconjunctivitis
Adenoviral keratoconjunctivitis, also known as Keratoconjunctivitis epidemica is a common and highly contagious viral infection of the eye.
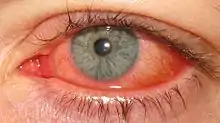
The clinical course of (AKC) should be divided into an acute phase with conjunctival inflammation of varying intensity with or without corneal involvement and a chronic phase with corneal opacities. AKC is caused by many different serotypes and is highly contagious during the acute phase.
Epidemiology
The economic and social price of AKC as a community epidemic is high.
Clinical signs
Corneal opacities, the hallmark of the chronic phase, are usually self-limited. They may occur as numular keratitis.
Treatment
Topical steroids should be avoided because they prolong viral replication, frequently lead to long-lasting dry eye symptoms, and corneal opacities almost always recur after discontinuation of topical steroids. There is currently no effective and clinically applicable topical antiviral agent for the treatment of the acute phase of AKC. Topical cidofovir is the first antiviral agent which has effectively reduced the incidence of corneal opacities, but local toxicity rules out its clinical application. Recently, NMSO3, a sulfated sialyl lipid, has demonstrated a greater antiviral potency against adenovirus in vitro than cidofovir exhibiting minimal cytotoxicity.[1] Topical cyclosporin A (CsA) appears to be effective in the treatment of persistent corneal opacities. Topical interferon might be effective as a prophylaxis of infection. Topical interferon is currently not commercially available due to unsettled patent issues.
Prevention
Adequate infection control measures should be followed as prevention and to reduce epidemic AKC outbreaks.[2]
References
- Kaneko H; et al. (2001). "Antiviral activity of NMSO3 against adenovirus in vitro". Antiviral Res. 52 (3): 281–8. doi:10.1016/s0166-3542(01)00167-x. PMID 11675145.
- Reinhard, Thomas; Larkin, Frank (28 January 2006). Cornea and External Eye Disease. google.com.au. ISBN 9783540312260. Retrieved 22 March 2015.
