Allophanic acid
Allophanic acid is the organic compound with the formula H2NC(O)NHCO2H. It is a carbamic acid, the carboxylated derivative of urea. Biuret can be viewed as the amide of allophanic acid. The compound can be prepared by treating urea with sodium bicarbonate:[1]
- H2NC(O)NH2 + NaHCO3 → H2NC(O)NHCO2H + NaOH
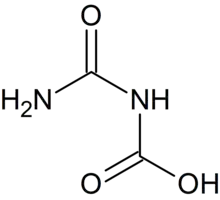 | |
| Names | |
|---|---|
| IUPAC name
carbamoylcarbamic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 1756623 | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H4N2O3 | |
| Molar mass | 104.065 g·mol−1 |
| Appearance | white solid |
| Melting point | 114 °C (237 °F; 387 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The anionicconjugate base, H2NC(O)NHCO2−, is called allophanate. Salt of this anion have been characterized by X-ray crystallography.[2][3] The anion allophonate is the substrate for the enzyme allophanate hydrolase.
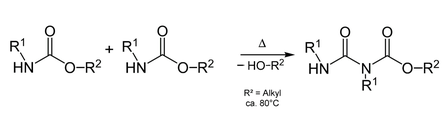
Allophonate esters arise from the condensation of carbamates.
References
- Karachinskii, S. V.; Dragalov, V. V.; Chimishkyan, A. L.; Tsvetkov, V. Yu. (1987). "Reaction of urea with alkali metal carbonates". Zhurnal Organicheskoi Khimii. 23: 93–6.
- Mak, Thomas C. W.; Yip, Wai Hing; Li, Qi (1995). "Novel Hydrogen-Bonded Host Lattices Built of Urea and the Elusive Allophanate Ion". Journal of the American Chemical Society. 117 (48): 11995–11996. doi:10.1021/ja00153a022.
- Li, Qi; Mak, Thomas C. W. (1996). "A Novel Inclusion Compound Consolidated by Host-host and Host-guest Hydrogen Bonding: (2-hydro-xyethyl)trimethylammonium Ions Included in a Channel Host Lattice Built of Urea Molecules and Allophanate Ions". Supramolecular Chemistry. 8: 73–80. doi:10.1080/10610279608233970.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.