Biuret
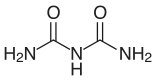
Biuret is a chemical compound with the chemical formula [H2NC(O)]2NH. It is a white solid that is soluble in hot water. The term "biuret" also describes a family of organic compounds with the functional group -(HN-CO-)2N-. Thus, dimethyl biuret is [Me(H)NC(O)]2NH. A variety of organic derivatives are known. Also known as carbamylurea, it results from the condensation of two equivalents of urea. As such, it is an undesirable impurity in urea-based fertilizers. As biuret is toxic to plants, its percentage in fertilizers must be kept low.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Imidodicarbonic diamide[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 1703510 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.236 |
| EC Number |
|
| 49702 | |
| KEGG | |
| MeSH | Biuret |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5N3O2 | |
| Molar mass | 103.081 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Density | 1.467 g/cm3 |
| Melting point | 190 °C (decomposes) |
| Thermochemistry | |
Heat capacity (C) |
131.3 J K−1 mol−1 |
Std molar entropy (S |
146.1 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−565.8–−561.6 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−940.1–−935.9 kJ mol−1 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| Related compounds | |
Related compounds |
urea, triuret, cyanuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and structure
The parent compound can be prepared by heating urea above the melting point at which temperature ammonia is expelled:[3]
- 2 CO(NH2)2 → H2N-CO-NH-CO-NH2 + NH3
Under related conditions, pyrolysis of urea affords triuret ((H2N-CO-NH)2CO).[3] In general, organic biurets (those with alkyl or aryl groups in place of one or more H atoms) are prepared by trimerization of isocyanates. For example, the trimer of 1,6-hexamethylene diisocyanate is also known as HDI-biuret.
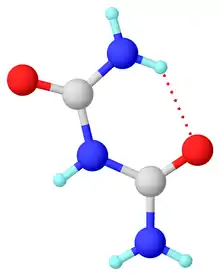
In the anhydrous material, the molecule is planar and unsymmetrical in the solid state owing to intramolecular hydrogen bonding. The terminal C-N distances of 1.327 and 1.334 Å are shorter than the internal C-N distances of 1.379 and 1.391 Å. The C=O bond distances 1.247 and 1.237 Å. It crystallizes from water as the hydrate.[4]
Applications
Biuret is also used as a non-protein nitrogen source in ruminant feed,[5] where it is converted into protein by gut microorganisms.[6] It is less favored than urea, due to its higher cost and lower digestibility[7] but the latter characteristic also slows down its digestion and so decreases the risk of ammonia toxicity.[7][8]
Biuret test
The biuret test is a chemical test for proteins and polypeptides. It is based on the biuret reagent, a blue solution that turns violet upon contact with proteins, or any substance with peptide bonds. The test and reagent do not actually contain biuret; they are so named because both biuret and proteins have the same response to the test.
History
Biuret was first prepared and studied by Gustav Heinrich Wiedemann (1826 - 1899) for his doctoral dissertation, which was submitted in 1847. His findings were reported in several articles.[9][10][11][12]
Related compounds
- Cyanuric acid
- Allophanic acid, the carboxylic acid derivative of biuret
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 866. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Scifinder, version 2007.1; Chemical Abstracts Service: Columbus, OH; RN 108-19-0 (accessed June 15, 2012)
- Meessen, J. H.; Petersen, H. "Urea". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_333.
- E. W. Hughes, H. Yakel, H. C. Freeman (1961). "The Crystal Structure of Biuret Hydrate". Acta Crystallogr. 14 (4): 345–352. doi:10.1107/S0365110X61001194.CS1 maint: uses authors parameter (link)
- Beef cattle feed, Encyclopædia Britannica Online
- Kunkle, B.; Fletcher, J.; Mayo, D. (2013). "Florida Cow-Calf Management, 2nd Edition - Feeding the Cow Herd". IFAS Extension, University of Florida. Publication #AN117.
- Oltjen, R. R.; Williams, E. E.; Slyter, L. L.; Richardson, G. V. (1969). "Urea versus biuret in a roughage diet for steers". Journal of Animal Science. 29 (5): 816–822. doi:10.2527/jas1969.295816x. PMID 5391979.
- Fonnesbeck, P. V.; Kearl, L. C.; Harris, L. E. (1975). "Feed Grade Biuret as a Protein Replacement for Ruminants. A Review". Journal of Animal Science. 40 (6): 1150–1184. doi:10.2527/jas1975.4061150x.
- Wiedemann, G. (1848). "Ueber ein neues Zersetzungsproduct des Harnstoffs" [On a new decomposition product of urea]. Annalen der Physik. 150 (5): 67–84. Bibcode:1848AnP...150...67W. doi:10.1002/andp.18491500508.
- Wiedemann, G. (1847). "Neues Zersetzungsproduct des Harnstoffs" [New decomposition product of urea]. Journal für Praktische Chemie. 42 (3–4): 255–256. doi:10.1002/prac.18470420134. This notice reports that biuret reacts with alkaline copper sulfate to produce a red solution -- the so-called "Biuret test"
- Wiedemann, G. (1848). "Ueber eine neue, aus dem Harnstoff entstehende Verbindung" [On a new compound arising from urea]. Journal für Praktische Chemie. 43 (5): 271–280. doi:10.1002/prac.18480430133.
- Wiedemann, G. (1848). "Biuret. Zersetzungsprodukt des Harnstoffs" [Biuret: decomposition product of urea]. Justus Liebig's Annalen der Chemie. 68 (3): 323–326. doi:10.1002/jlac.18480680318.