Aluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al(O-i-Pr)3, where i-Pr is the isopropyl group (–CH(CH3)2). This colourless solid is a useful reagent in organic synthesis.
12.png.webp) | |
| Names | |
|---|---|
| IUPAC name
Aluminium Isopropoxide | |
| Other names
Triisopropoxyaluminium Aluminium isopropanolate Aluminium sec-propanolate Aluminium triisopropoxide 2-Propanol aluminium salt AIP | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.265 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H21AlO3 | |
| Molar mass | 204.246 g·mol−1 |
| Appearance | white solid |
| Density | 1.035 g cm−3, solid |
| Melting point | Sensitive to purity: 138–142 °C (99.99+%) 118 °C (98+%)[1] |
| Boiling point | @10 Torr 135 °C (408 K) |
| Decomposes | |
| Solubility in isopropanol | Poor |
| Structure | |
| monoclinic | |
| Hazards | |
| Main hazards | Flammable (F) |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H228 | |
| P210, P240, P241, P280 | |
| NFPA 704 (fire diamond) | |
| Flash point | 16 °C (61 °F; 289 K) |
| Related compounds | |
Other cations |
Titanium isopropoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
A tetrameric structure of the crystalline material was verified by NMR spectroscopy and X-ray crystallography. The species is described by the formula Al[(μ-O-i-Pr)2Al(O-i-Pr)2]3.[2][3] The unique central Al is octahedral, and three other Al centers adopt tetrahedral geometry. The idealised point group symmetry is D3.
Preparation
This compound is commercially available. Industrially, it is prepared by the reaction between isopropyl alcohol and aluminium metal, or aluminium trichloride:
- 2 Al + 6 iPrOH → 2 Al(O-i-Pr)3 +3H2
- AlCl3 + 3 iPrOH → Al(O-i-Pr)3 + 3 HCl
The procedure entails heating a mixture of aluminium, isopropyl alcohol, with a small amount of mercuric chloride. The process occurs via the formation of an amalgam of the aluminium. A catalytic amount of iodine is sometimes added to initiate the reaction.[4] The industrial route does not use mercury.[5]
Reactions
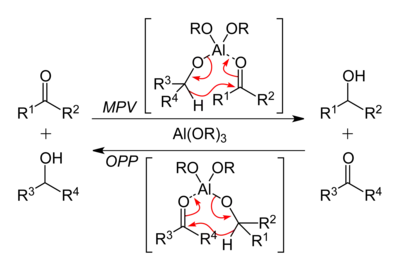
Aluminium isopropoxide is used in MPV reductions of ketones and aldehydes and the Oppenauer Oxidation of secondary alcohols.[6] In these reactions, it is assumed that the tetrameric cluster disagregates. It is used in the Tishchenko reaction.
Being a basic alkoxide, Al(O-i-Pr)3 has been also investigated as a catalyst for ring opening polymerization of cyclic esters.[7]
History
Aluminium isopropoxide was first reported in the master's thesis of the Russian organic chemist Vyacheslav Tishchenko (Вячеслав Евгеньевич Тищенко, 1861–1941), which was reprinted in the Journal of the Russian Physico-Chemical Society (Журнал Русского Физико-Химического Общества) of 1899.[8] This contribution included a detailed description of its synthesis, its peculiar physico-chemical behavior, and its catalytic activity in the Tishchenko reaction (catalytic transformation of aldehydes into esters). It was later found also to display catalytic activity as a reducing agent by Meerwein and Schmidt in the Meerwein–Ponndorf–Verley reduction ("MPV") in 1925.[9][10] The reverse of the MPV reaction, oxidation of an alcohol to a ketone, is termed the Oppenauer oxidation. The original Oppenauer oxidation employed aluminium butoxide in place of the isopropoxide.[11]
Related compounds
Aluminium tert-butoxide is a dimer [(t-Bu-O)2Al(μ-O-t-Bu)]2.[12] It is prepared analogously to the isopropoxide.[13]
References
- Ishihara, K.; Yamamoto, H. (2001). "Aluminum Isopropoxide". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.ra084.
- Folting, K.; Streib, W. E.; Caulton, K. G.; Poncelet, O.; Hubert-Pfalzgraf, L. G. (1991). "Characterization of aluminum isopropoxide and aluminosiloxanes". Polyhedron. 10 (14): 1639–46. doi:10.1016/S0277-5387(00)83775-4.
- Turova, N. Y.; Kozunov, V. A.; Yanovskii, A. I.; Bokii, N. G.; Struchkov, Yu T.; Tarnopolskii, B. L. (1979). "Physico-chemical and structural investigation of aluminium isopropoxide." J. Inorg. Nucl. Chem. 41(1): 5-11, doi:10.1016/0022-1902(79)80384-X.
- Young, W.; Hartung, W.; Crossley, F. (1936). "Reduction of Aldehydes with Aluminum Isopropoxide". J. Am. Chem. Soc. 58: 100–102. doi:10.1021/ja01292a033.
- Otto Helmboldt; L. Keith Hudson; Chanakya Misra; Karl Wefers; Wolfgang Heck; Hans Stark; Max Danner; Norbert Rösch. "Aluminum Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_527.pub2.
- Eastham, Jerome F.; Teranishi, Roy (1955). "Δ4-Cholesten-3-one". 35: 39. doi:10.15227/orgsyn.035.0039. Cite journal requires
|journal=(help) - Tian, D.; Dubois, Ph.; Jérôme, R. (1997). "Macromolecular Engineering of Polylactones and Polylactides. 22. Copolymerization of ε-Caprolactone and 1,4,8-Trioxaspiro[4.6]-9-undecanone Initiated by Aluminum Isopropoxide". Macromolecules. 30 (9): 2575–2581. doi:10.1021/ma961567w.
- Тищенко, B. E. (Tishchenko, V. E.) (1899). "Действие амальгамированного алюминия на алкоголь. Алкоголятов алюминия, их свойства и реакции" [Effect of amalgamated aluminium on alcohol. Aluminium alkoxides, their properties and reactions.]. Журнал Русского Физико-Химического Общества (Journal of the Russian Physico-Chemical Society) (in Russian). 31: 694–770.CS1 maint: multiple names: authors list (link)
- Meerwein, H.; Schmidt, R. (1925). "Ein neues Verfahren zur Reduktion von Aldehyden und Ketonen" [A new procedure for the reduction of aldehydes and ketones]. Justus Liebigs Ann. Chem. (in German). 444: 221–238. doi:10.1002/jlac.19254440112.
- Wilds, A. L. (1944). "Reduction with Aluminum Alkoxides (The Meerwein-Ponndorf-Verley Reduction)". Org. React. 2 (5): 178–223. doi:10.1002/0471264180.or002.05.
- Oppenauer, R. V. (1937). "Eine Methode der Dehydrierung von Sekundären Alkoholen zu Ketonen. I. Zur Herstellung von Sterinketonen und Sexualhormonen" [Dehydration of secondary alcohols to ketones. I. Preparation of sterol ketones and sex hormones]. Recl. Trav. Chim. Pays-Bas (in German). 56 (2): 137–144. doi:10.1002/recl.19370560206.
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- Wayne, Winston; Adkins, Homer (1941). "Aluminum tert-Butoxide". 21: 8. doi:10.15227/orgsyn.021.0008. Cite journal requires
|journal=(help)
