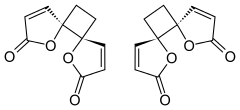
Anemonin
Anemonin is a compound found in plants of the buttercup family (Ranunculaceae). It is the dimerization product of the toxin protoanemonin[2] and is easily hydrolysed to a dicarboxylic acid.[3]
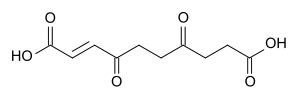
4,7-Dioxo-2-decenedioic acid, the hydrolysis product of anemonin
 | |
 | |
| Names | |
|---|---|
| IUPAC names
trans-4,7-Dioxadispiro[4.0.46.25]dodeca-1,9-diene-3,8-dione trans-1,7-Dioxadispiro[4.0.4.2]dodeca-3,9-diene-2,8-dione[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8O4 | |
| Molar mass | 192.170 g·mol−1 |
| Appearance | Colourless, odourless solid |
| Density | 1.45g/cm3 |
| Melting point | 158[1] °C (316 °F; 431 K) |
| Boiling point | 535.7 °C (996.3 °F; 808.9 K) @ 760mmHg |
| low | |
| Solubility in chloroform | very soluble[1] |
| Hazards | |
| Flash point | 300.7 °C (573.3 °F; 573.8 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
150 mg·kg−1 (mouse, i. p.) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The name of the substance comes from the plant genus Anemone, from which it was first identified.[4]
Potential uses
Antispasmodic and analgetic properties have been described.[5]
The compound appears to inhibit pigmentation synthesis, and has therefore been suggested as a potential candidate for cosmetic use.[6]
References
- William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. p. 3-26. ISBN 978-1-4987-5429-3.
- List, PH; Hörhammer, L, eds. (1979). Hagers Handbuch der pharmazeutischen Praxis (in German) (4 ed.). Springer Verlag. ISBN 3-540-07738-3.
- "Aktuelles aus der Natur" (PDF) (in German). TU Graz. 2 April 2009. p. 4. Retrieved 27 November 2010.
- Chemie der organischen Verbindungen, Carl Löwig (in German)
- Anemonin, Wissenschaft online (in German)
- Huang, Yen-Hua; Lee, Tzong-Huei; Chan, Kuei-Jung; Hsu, Feng-Lin; Wu, Yu-Chih; Lee, Mei-Hsien (February 2008). "Anemonin is a natural bioactive compound that can regulate tyrosinase-related proteins and mRNA in human melanocytes". Journal of Dermatological Science. 49 (2): 115–123. doi:10.1016/j.jdermsci.2007.07.008. PMID 17766092.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.