Archaerhodopsin
Archaerhodopsin proteins are a family of retinal-containing photoreceptors found in the archaea genera Halobacterium and Halorubrum. Like the homologous bacteriorhodopsin (bR) protein, archaerhodopsins harvest energy from sunlight to pump H+ ions out of the cell, establishing a proton motive force that is used for ATP synthesis. They have some structural similarities to the mammalian GPCR protein rhodopsin, but are not true homologs.
Archaerhodopsins differ from bR in that the claret membrane, in which they are expressed, includes bacterioruberin, a second chromophore thought to protect against photobleaching. bR also lacks the omega loop structure that has been observed at the N-terminus of the structures of several archaerhodopsins.
Mutants of Archaerhodopsin-3 (AR3) are widely used as tools in optogenetics for neuroscience research.[1]
Etymology
The term archaerhodopsin is a portmanteau of archaea (the domain in which the proteins are found) and rhodopsin (a photoreceptor responsible for vision in the mammalian eye).[2]
Members of the archaerhodopsin family
Seven members of the archaerhodopsin family have been identified to date.
| Name | Abbr. | Organism | GenBank | UniProt | PDB | Ref. |
|---|---|---|---|---|---|---|
| Archaerhodopsin-1 | AR1 | Halobacterium sp. Aus-1 | J05165 | P69052 | 1UAZ | [2] |
| Archaerhodopsin-2 | AR2 | Halobacterium sp. Aus-2 | S56354 | P29563 | 3WQJ | [5] |
| Archaerhodopsin-3 | AR3 or Arch | Halorubrum sodomense | GU045593 | P96787 | 6S6C | [6] |
| Archaerhodopsin-4 | AR4 | Halobacterium sp. xz515 | AF306937 | [7] | ||
| Archaerhodopsin-BD1 | AR-BD1 or HxAR | Halorubrum xinjiangense | AY510709 | Q6R5N7 | [8] | |
| Archaerhodopsin-He | HeAr | Halorubrum ejinorense | LC073751 | [9] | ||
| Archaerhodopsin-TP009 | AR-TP009 | Halorubrum sp. TP009 | [10] |
Archaerhodopsins-1 and -2 (AR1 and AR2)
Archaerhodopsin 1 and 2 (AR1 and AR2) were the first archaerhodopsins to be identified and are expressed by Halobacterium sp. Aus-1 and Aus-2 respectively. Both species were first isolated in Western Australia in the late 1980s.[2][5][11] The crystal structures of both proteins were solved by Kunio Ihara, Tsutomo Kouyama and co-workers at Nagoya University, together with collaborators at the Spring-8 synchrotron.[12]
Archaerhodopsin-3 (AR3 or Arch)
AR3 is expressed by Halorubrum sodomense.[6] The organism was first identified in the Dead Sea in 1980 and requires a higher concentration of Mg2+ ions for growth than related halophiles.[13] The aop3 gene was cloned by Ihara and colleagues at Nagoya University in 1999 and the protein was found to share 59% sequence identity with bacteriorhodopsin.[6] The crystal structure of AR3 was solved by Anthony Watts at Oxford University and Isabel Moraes at the National Physical Laboratory, together with collaborators at Diamond Light Source.[14]
Mutants of Archaerhodopsin-3 (AR3) are widely used as tools in optogenetics for neuroscience research.[1]
AR3 has recently been introduced as a fluorescent voltage sensor.[15]
Archaerhodopsin-4 (AR4)
AR4 is expressed in Halobacterium species xz 515. The organism was first identified in a salt lake in Tibet.[7][16] The gene encoding AR4 was identified by H Wang and colleagues in 2000.[17] In most bacteriorhodopsin homologs, H+ release to the extracellular medium takes place before a replacement ion is taken up from the cytosolic side of the membrane, however under the acidic conditions found in the organism’s native habitat, the order of these stages in the AR4 photocycle is reversed.[18]
Archaerhodopsin-BD1 (AR-BD1)
AR-BD1 (also known as HxAR) is expressed by Halorubrum xinjiangense.[8] The organism was first isolated from Xiao-Er-Kule Lake in Xinjiang, China.[19]
Archaerhodopsin-He (HeAr)
HeAR is expressed by Halorubrum ejinorense.[9] The organism was first isolated from Lake Ejinor in Inner Mongolia, China.[20]
Archaerhodopsin-TP009 (AR-TP009 or ArchT)
AR-TP009 is expressed by Halorubrum sp. TP009. Its ability to act as a neural silencer has been investigated in mouse cortical pyramidal neurons.[10]
General features
Occurrence
Like other members of the microbial rhodopsin family, archaerhodopsins are expressed in specialised, protein-rich domains of the cell surface membrane, commonly called the claret membrane. In addition to ether lipids, the claret membrane contains bacterioruberin, (a 50-carbon carotenoid pigment) which is thought to protect against photobleaching. Atomic force microscope images of the claret membranes of several archaerhodopsins, show that the proteins are trimeric and are arranged in a hexagonal lattice.[21] Bacterioruberin has also been implicated in oligomerisation and may facilitate protein-protein interactions in the native membrane.[22][23]
Function
Archaerhodopsins are active transporters, using the energy from sunlight to pump H+ ions out of the cell to generate a proton motive force that is used for ATP synthesis. Removal of the retinal cofactor (e.g. by treatment with hydroxylamine) abolishes the transporter function and dramatically alters the absorption spectra of the proteins. The proton pumping ability of AR3 has been demonstrated in recombinant E. coli cells[24] and of AR4 in liposomes.[18]
In the resting or ground state of archaerhodopsin, the bound retinal is in the all-trans form, but on absorption of a photon of light, it isomerizes to 13-cis. The protein surrounding the chromophore reacts to the change of shape and undergoes an ordered sequence of conformational changes, which are collectively known as the photocycle. These changes alter the polarity of the local environment surrounding titratable amino acid side chains inside the protein, enabling H+ to be pumped from the cytoplasm to the extracellular side of the membrane. The intermediate states of the photocycle may be identified by their absorption maxima.[18][25]
Structures
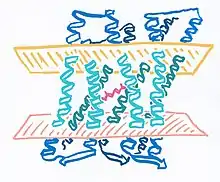
Crystal structures of the resting or ground states of AR1 (3.4 Å resolution) and AR2 (1.8 Å resolution) have been deposited in the Protein Data Bank.[12][26] Both proteins have seven transmembrane α-helices and a two-stranded extracellular-facing β-sheet. Retinal is covalently bonded via Schiff base to a lysine residue on helix G.[12][note 1] The conserved DLLxDGR sequence, close to the extracellular-facing N-terminus of both proteins, forms a tightly curved omega loop that has been implicated in bacterioruberin binding.[22]
Use in research
Archaerhodopsins drive the hyperpolarization of the cell membrane by secreting protons in presence of light, thereby inhibiting action potential firing of neurons.[27] This process is associated to an increase in extracellular pH linked to the activity of these proteins. These characteristics allow for Archaerhodopsins to be commonly used tools for optogenetic studies as they behave as transmission inhibition factors in presence of light.[28] When expressed within intracellular membranes, the proton pump activity increases the cytosolic pH, this functionality can be used for optogenetic acidification of lysosomes and synaptic vesicles when targeted to these organelles.[29]
History
In the 1960s, a light driven proton pump was discovered in Halobacterium salinarum, and called Bacteriorhodopsin. Over the following years, there were various studies of the membrane of H. salinarum to determine the mechanism of these light-driven proton pumps.
In 1988, another Manabu Yoshida's group at Osaka University reported a novel light-sensitive proton pump from a strain of Halobacterium which they termed Archaerhodopsin.[2] A year later, the same group reported isolating the gene that encodes Archaerhodopsin.[11][30]
Notes
- Helix G is the nearest transmembrane helix to the C-terminus.
References
- Flytzanis NC, Bedbrook CN, Chiu H, Engqvist MK, Xiao C, Chan KY, Sternberg PW, Arnold FH, Gradinaru V (2014). "Archaerhodopsin variants with enhanced voltage-sensitive fluorescence in mammalian and Caenorhabditis elegans neurons". Nature Communications. 5: 4894. Bibcode:2014NatCo...5.4894F. doi:10.1038/ncomms5894. PMC 4166526. PMID 25222271.
- Mukohata Y, Sugiyama Y, Ihara K, Yoshida M (March 1988). "An Australian halobacterium contains a novel proton pump retinal protein: archaerhodopsin". Biochemical and Biophysical Research Communications. 151 (3): 1339–45. doi:10.1016/S0006-291X(88)80509-6. PMID 2833260.
- Archaea in Wiktionary The free dictionary
- Rhodopsin in Wiktionary The free dictionary
- Uegaki K, Sugiyama Y, Mukohata Y (April 1991). "Archaerhodopsin-2, from Halobacterium sp. aus-2 further reveals essential amino acid residues for light-driven proton pumps". Archives of Biochemistry and Biophysics. 286 (1): 107–10. doi:10.1016/0003-9861(91)90014-A. PMID 1654776.
- Ihara K, Umemura T, Katagiri I, Kitajima-Ihara T, Sugiyama Y, Kimura Y, Mukohata Y (January 1999). "Evolution of the archaeal rhodopsins: evolution rate changes by gene duplication and functional differentiation". Journal of Molecular Biology. 285 (1): 163–74. doi:10.1006/jmbi.1998.2286. PMID 9878396.
- Li Q, Sun Q, Zhao W, Wang H, Xu D (June 2000). "Newly isolated archaerhodopsin from a strain of Chinese halobacteria and its proton pumping behavior". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1466 (1–2): 260–6. doi:10.1016/S0005-2736(00)00188-7. PMID 10825447.
- Geng X, Dai G, Chao L, Wen D, Kikukawa T, Iwasa T (2019). "Two consecutive polar amino acids at the end of helix E are important for fast turnover of the archaerhodopsin photocycle". Photochemistry and Photobiology. 95: 980–989. doi:10.1111/php.13072. PMID 30548616.
- Chaoluomeng, Dai G, Kikukawa T, Ihara K, Iwasa T (2015). "Microbial Rhodopsins of Halorubrum Species Isolated From Ejinoor Salt Lake in Inner Mongolia of China". Photochem. Photobiol. Sci. 14 (11): 1974–82. doi:10.1039/c5pp00161g. PMID 26328780.
- Xue H, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES (2011). "A high-light sensitivity optical neural silencer: Development and application to optogenetic control of non-human primate cortex". Frontiers in Systems Neuroscience. 5 (18): 1–8. doi:10.3389/fnsys.2011.00018. PMC 3082132. PMID 21811444.
- Sugiyama Y, Maeda M, Futai M, Mukuhata Y (1989). "Isolation of a Gene That Encodes a New Retinal Protein, Archaerhodopsin, From Halobacterium Sp. Aus-1". J. Biol. Chem. 264 (35): 20859–62. PMID 2592356.
- Enami N, Yoshimura K, Murakami M, Okumura H, Ihara K, Kouyama T (2006). "Crystal Structures of Archaerhodopsin-1 and -2: Common structural motif in archaeal light-driven proton pumps". J. Mol. Biol. 358 (3): 675–685. doi:10.1016/j.jmb.2006.02.032. PMID 16540121.
- Oren A (1983). "Halobacterium sodomense sp. nov. a Dead Sea Halobacterium with an extremely high magnesium requirement". International Journal of Systematic Bacteriology. 33 (2): 381–386. doi:10.1099/00207713-33-2-381.
- Bada Juarez JF, Judge PJ, Adam S, Axford D, Vinals J, Birch J, Kwan TO, Hoi KK, Yen HY, Vial A, Milhiet PE, Robinson CV, Schapiro I, Moraes I, Watts A (2021). "Structures of the archaerhodopsin 3 transporter reveal that disordering of internal water networks underpins receptor sensitization". Nature Communications. 12: 629. doi:10.1038/s41467-020-20596-0. PMC 7840839. PMID 33504778.
- Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE (November 2011). "Optical recording of action potentials in mammalian neurons using a microbial rhodopsin". Nature Methods. 9 (1): 90–5. doi:10.1038/nmeth.1782. PMC 3248630. PMID 22120467.
- Wang Y, Ma D, Zhao Y, Ming M, Wu J, Ding J (2012). "Light-driven proton pumps of archaerhodopsin and bacteriorhodopsin & polymer-matrix composite materials of those functional proteins". Acta Polymerica Sinica. 12 (7): 698–713. doi:10.3724/SP.J.1105.2012.12051.
- Wang, S. Zhan, Q. Sun, D. Xu, W. Zhao, W. Huang, Q. Li., 2000. Primary structure of helix C to helix G of a new retinal protein in H.sp.xz515. Chin. Sci. Bull., 45: 1108-1113.
- Ming M, Lu M, Balashov SP, Ebrey TG, Li Q, Ding J (2006). "pH Dependence of Light-Driven Proton Pumping by an Archaerhodopsin From Tibet: Comparison With Bacteriorhodopsin". Biophys. J. 90 (9): 3322–32. Bibcode:2006BpJ....90.3322M. doi:10.1529/biophysj.105.076547. PMC 1432102. PMID 16473896.
- Feng J, Zhou PJ, Liu SJ (2004). "Halorubrum xinjiangense sp. nov., a novel halophile isolated from saline lakes in China". International Journal of Systematic and Evolutionary Microbiology. 54 (5): 1789–1791. doi:10.1099/ijs.0.63209-0. PMID 15388744.
- Castillo AM, Gutiérrez MC, Kamekura M, Xue Y, Ma Y, Cowan DA, Jones BE, Grant WD, Ventosa A (2007). "Halorubrum Ejinorense Sp. Nov., Isolated From Lake Ejinor, Inner Mongolia, China". International Journal of Systematic and Evolutionary Microbiology. 57 (11): 2538–2542. doi:10.1099/ijs.0.65241-0. PMID 17978215.
- Tang L, Sun Q, Li Q, Huang Y, Wei Q, Zhang Y, Hu J, Zhang Z (2001). "Imaging bacteriorhodopsinlike molecules of claretmembranes from Tibet halobacteria xz515 by atomic force microscope". Chinese Science Bulletin. 46 (22): 1897–1900. Bibcode:2001ChSBu..46.1897T. doi:10.1007/BF02901167. S2CID 96378194.
- Yoshimura K, Kouyama T (2008). "Structural Role of Bacterioruberin in the Trimeric Structure of Archaerhodopsin-2". Journal of Molecular Biology. 375 (5): 1267–81. doi:10.1016/j.jmb.2007.11.039. PMID 18082767.
- Chao S, Ding X, Cui H, Yang Y, Chen S, Watts A, Zhao X (2018). "In Situ Study of the Function of Bacterioruberin in the Dual-Chromophore Photoreceptor Archaerhodopsin-4". Angew. Chem. 57 (29): 8937–8941. doi:10.1002/anie.201803195. PMID 29781190.
- Gunapathy S, Kratx S, Chen Q, Hellingwerf K, de Groot H, Rothschild K, de Grip W (2019). "Redshifted and Near-infrared Active Analog Pigments Based upon Archaerhodopsin-3". Photochemistry and Photobiology. 95 (4): 959–968. doi:10.1111/php.13093. PMC 6849744. PMID 30860604.
- Geng X, Dai G, Chao L, Wen D, Kikukawa T, Iwasa T (2019). "Two Consecutive Polar Amino Acids at the End of Helix E are Important for Fast Turnover of the Archaerhodopsin Photocycle". Photochemistry and Photobiology. 95 (4): 980–989. doi:10.1111/php.13072. PMID 30548616.
- Kouyama T, Fujii R, Kanada S, Nakanishi T, Chan SK, Midori M (2014). "Structure of archaerhodopsin-2 at 1.8 Å Resolution". Acta Crystallogr D. 70 (10): 2692–701. doi:10.1107/S1399004714017313. PMC 4188009. PMID 25286853.
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES (January 2010). "High-performance genetically targetable optical neural silencing by light-driven proton pumps". Nature. 463 (7277): 98–102. Bibcode:2010Natur.463...98C. doi:10.1038/nature08652. PMC 2939492. PMID 20054397.
- El-Gaby M, Zhang Y, Wolf K, Schwiening CJ, Paulsen O, Shipton OA (2016). "Archaerhodopsin Selectively and Reversibly Silences Synaptic Transmission through Altered pH". Cell Reports. 16 (8): 2259–68. doi:10.1016/j.celrep.2016.07.057. PMC 4999416. PMID 27524609.
- Rost BR, Schneider F, Grauel MK, Wozny C, Bentz C, Blessing A, Rosenmund T, Jentsch TJ, Schmitz D, Hegemann P, Rosenmund C (December 2015). "Optogenetic acidification of synaptic vesicles and lysosomes". Nature Neuroscience. 18 (12): 1845–1852. doi:10.1038/nn.4161. PMC 4869830. PMID 26551543.
- Oren, A (1994). "Enzyme diversity in halophilic archaea" (PDF). Microbiología. 10 (3): 217–228. PMID 7873098. Archived from the original (PDF) on 4 July 2012. Retrieved 8 April 2018.