Becquerel
The becquerel (English: /bɛkəˈrɛl/; symbol: Bq) is the SI derived unit of radioactivity. One becquerel is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. For applications relating to human health this is a small quantity,[1] and SI multiples of the unit are commonly used.[2]
| Becquerel | |
|---|---|
| Unit system | SI derived unit |
| Unit of | Activity |
| Symbol | Bq |
| Named after | Henri Becquerel |
| Conversions | |
| 1 Bq in ... | ... is equal to ... |
| rutherford | 10−6 Rd |
| curie | 2.703×10−11 Ci ≅ 27 pCi |
| SI base unit | s−1 |
The becquerel is named after Henri Becquerel, who shared a Nobel Prize in Physics with Pierre and Marie Skłodowska Curie in 1903 for their work in discovering radioactivity.[3]
Definition
1 Bq = 1 s−1
A special name was introduced for the reciprocal second (s−1) to represent radioactivity to avoid potentially dangerous mistakes with prefixes. For example, 1 µs−1 would mean 106 disintegrations per second: 1·(10−6 s)−1 = 106 s−1,[4] whereas 1 µBq would mean 1 disintegration per 1 million seconds. Other names considered were hertz (Hz), a special name already in use for the reciprocal second, and Fourier (Fr).[4] The hertz is now only used for periodic phenomena.[5] Whereas 1 Hz is 1 cycle per second, 1 Bq is 1 aperiodic radioactivity event per second.
The gray (Gy) and the becquerel (Bq) were introduced in 1975.[6] Between 1953 and 1975, absorbed dose was often measured in rads. Decay activity was measured in curies before 1946 and often in rutherfords between 1946[7] and 1975.
Unit capitalization and prefixes
As with every International System of Units (SI) unit named for a person, the first letter of its symbol is uppercase (Bq). However, when an SI unit is spelled out in English, it should always begin with a lowercase letter (becquerel)—except in a situation where any word in that position would be capitalized, such as at the beginning of a sentence or in material using title case.[8]
Like any SI unit, Bq can be prefixed; commonly used multiples are kBq (kilobecquerel, 103 Bq), MBq (megabecquerel, 106 Bq, equivalent to 1 rutherford), GBq (gigabecquerel, 109 Bq), TBq (terabecquerel, 1012 Bq), and PBq (petabecquerel, 1015 Bq). Large prefixes are common for practical uses of the unit.
Calculation of radioactivity
For a given mass (in grams) of an isotope with atomic mass (in g/mol) and a half-life of (in s), the radioactivity can be calculated using:
With = 6.02214076×1023 mol−1, the Avogadro constant.
Since is the number of moles (), the amount of radioactivity can be calculated by:
For instance, on average each gram of potassium contains 0.000117 gram of 40K (all other naturally occurring isotopes are stable) that has a of 1.277×109 years = 4.030×1016 s,[9] and has an atomic mass of 39.964 g/mol,[10] so the amount of radioactivity associated with a gram of potassium is 30 Bq.
Examples
For practical applications, 1 Bq is a small unit. For example, the roughly 0.0169 g of potassium-40 present in a typical human body produces approximately 4,400 disintegrations per second or 4.4 kBq of activity.[11]
The global inventory of carbon-14 is estimated to be 8.5×1018 Bq (8.5 EBq, 8.5 exabecquerel).[12] The nuclear explosion in Hiroshima (an explosion of 16 kt or 67 TJ) is estimated to have produced 8×1024 Bq (8 YBq, 8 yottabecquerel).[13]
These examples are useful for comparing the amount activity of these radioactive materials but should not be confused with the amount of exposure to ionizing radiation that these materials represent. The level of exposure and thus the absorbed dose received are what should be considered when assessing the effects of ionizing radiation on humans.
Relation to the curie
The becquerel succeeded the curie (Ci),[14] an older, non-SI unit of radioactivity based on the activity of 1 gram of radium-226. The curie is defined as 3.7×1010 s−1, or 37 GBq.[4][15]
Conversion factors:
- 1 Ci = 3.7×1010 Bq = 37 GBq
- 1 μCi = 37,000 Bq = 37 kBq
- 1 Bq = 2.7×10−11 Ci = 2.7×10−5 μCi
- 1 MBq = 0.027 mCi
Relation to other radiation-related quantities
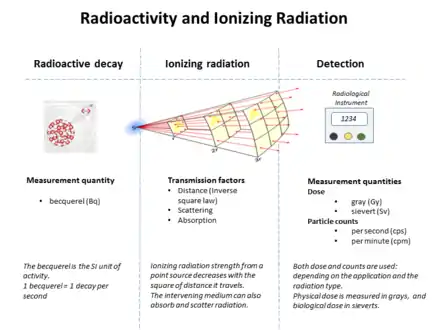
The following table shows radiation quantities in SI and non-SI units. WR (formerly 'Q' factor) is a factor that scales the biological effect for different types of radiation, relative to x-rays. (e.g. 1 for beta radiation, 20 for alpha radiation, and a complicated function of energy for neutrons) In general conversion between rates of emission, the density of radiation, the fraction absorbed, and the biological effects, requires knowledge of the geometry between source and target, the energy and the type of the radiation emitted, among other factors. [16]
| Quantity | Unit | Symbol | Derivation | Year | SI equivalence |
|---|---|---|---|---|---|
| Activity (A) | becquerel | Bq | s−1 | 1974 | SI unit |
| curie | Ci | 3.7 × 1010 s−1 | 1953 | 3.7×1010 Bq | |
| rutherford | Rd | 106 s−1 | 1946 | 1,000,000 Bq | |
| Exposure (X) | coulomb per kilogram | C/kg | C⋅kg−1 of air | 1974 | SI unit |
| röntgen | R | esu / 0.001293 g of air | 1928 | 2.58 × 10−4 C/kg | |
| Absorbed dose (D) | gray | Gy | J⋅kg−1 | 1974 | SI unit |
| erg per gram | erg/g | erg⋅g−1 | 1950 | 1.0 × 10−4 Gy | |
| rad | rad | 100 erg⋅g−1 | 1953 | 0.010 Gy | |
| Equivalent dose (H) | sievert | Sv | J⋅kg−1 × WR | 1977 | SI unit |
| röntgen equivalent man | rem | 100 erg⋅g−1 x WR | 1971 | 0.010 Sv | |
| Effective dose (E) | sievert | Sv | J⋅kg−1 × WR x WT | 1977 | SI unit |
| röntgen equivalent man | rem | 100 erg⋅g−1 x WR x WT | 1971 | 0.010 Sv |
See also
- Background radiation
- Banana equivalent dose
- Counts per minute
- Ionizing radiation
- Orders of magnitude (radiation)
- Radiation poisoning
- Relative biological effectiveness
- Rem (unit)
- Rutherford (unit)
- Sievert (biological dose equivalent of radiation)
References
- "Radioactivity : Radioactive Activity Doses". www.radioactivity.eu.com. Retrieved 20 February 2020.
- "Radiation Protection Guidance For Hospital Staff – Stanford Environmental Health & Safety". ehs.stanford.edu. Retrieved 20 February 2020.
- "BIPM - Becquerel". BIPM. Retrieved 2012-10-24.
- Allisy, A. (1995), "From the curie to the becquerel", Metrologia, 32 (6): 467–479, Bibcode:1995Metro..31..467A, doi:10.1088/0026-1394/31/6/006
- "BIPM - Table 3". BIPM. Retrieved 2015-07-19.
(d) The hertz is used only for periodic phenomena, and the becquerel is used only for stochastic processes in activity referred to a radionuclide.
- Harder, D (1976), "[The new radiologic units of measurement gray and becquerel (author's translation from the German original)]", Röntgen-Blätter, 29 (1): 49–52, PMID 1251122.
- Lind, SC (1946), "New units for the measurement of radioactivity", Science, 103 (2687): 761–762, Bibcode:1946Sci...103..761L, doi:10.1126/science.103.2687.761-a, PMID 17836457.
- "SI Brochure: The International System of Units (SI)". SI Brochure (8 ed.). BIPM. 2014.
- "Table of Isotopes decay data". Lund University. 1990-06-01. Retrieved 2014-01-12.
- "Atomic Weights and Isotopic Compositions for All Elements". NIST. Retrieved 2014-01-12.
- Radioactive human body — Harvard University Natural Science Lecture Demonstrations - Accessed October 2013
- G.R. Choppin, J.O.Liljenzin, J. Rydberg, "Radiochemistry and Nuclear Chemistry", 3rd edition, Butterworth-Heinemann, 2002. ISBN 978-0-7506-7463-8.
- Michael J. Kennish, Pollution Impacts on Marine Biotic Communities , CRC Press, 1998, p. 74. ISBN 978-0-8493-8428-8.
- It was adopted by the BIPM in 1975, see resolution 8 of the 15th CGPM meeting
- Resolution 7 of the 12th CGPM (1964)
- http://hps.org/publicinformation/ate/faqs/gammaandexposure.html
External links
| Look up becquerel in Wiktionary, the free dictionary. |
- Derived units on the International Bureau of Weights and Measures (BIPM) web site

