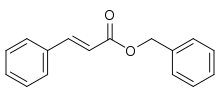
Benzyl cinnamate
Benzyl cinnamate is the chemical compound which is the ester derived from cinnamic acid and benzyl alcohol.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Benzyl (2E)-3-phenylprop-2-enoate | |
| Other names
Benzyl cinnamate Cinnamein Benzyl cinnamoate Benzyl 3-phenylpropenoate 3-Phenyl-2-propenoic acid phenylmethyl ester Cinnamic acid benzyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.827 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H14O2 | |
| Molar mass | 238.286 g·mol−1 |
| Appearance | White to pale yellow solid[1] |
| Melting point | 34–37 °C (93–99 °F; 307–310 K)[2] |
| Boiling point | 195–200 °C (383–392 °F; 468–473 K) 5 mmHg[2] |
| Insoluble[1] | |
| Solubility in ethanol | 125 g/L |
| Solubility in glycerin | Insoluble |
| Solubility in propylene glycol | Insoluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrence
Benzyl cinnamate occurs in Balsam of Peru and Tolu balsam, in Sumatra and Penang benzoin, and as the main constituent of copaiba balsam.[3]
Synthesis
Benzyl cinnamate can be prepared by heating benzyl chloride and excess sodium cinnamate in water to 100–115 °C or by heating sodium cinnamate with an excess of benzyl chloride in the presence of diethylamine.[3]
Uses
Benzyl cinnamate is used in heavy oriental perfumes and as a fixative.[4] It is used as a flavoring agent.[3]
References
- "Specifications for Flavourings". Food and Agriculture Organization.
- "Benzyl cinnamate". Sigma-Aldrich.
- George A. Burdock (2010), "BENZYL CINNAMATE", Fenaroli's Handbook of Flavor Ingredients (6th ed.), CRC Press, pp. 147–148
- Karl-Georg Fahlbusch; et al. (2007), "Flavors and Fragrances", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 59
External links
- Benzyl cinnamate at National Library of Medicine's Toxicology Data Network
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.