Bergman cyclization
The Bergman cyclization or Bergman reaction or Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen donor (Scheme 1).[1] It is the most famous and well-studied member of the general class of cycloaromatization reactions.[2] It is named for the American chemist Robert G. Bergman (b. 1942). The reaction product is a derivative of benzene.

| Bergman cyclization | |
|---|---|
| Named after | Robert George Bergman |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | bergman-cyclization |
| RSC ontology ID | RXNO:0000240 |
The reaction proceeds by a thermal reaction or pyrolysis (above 200 °C) forming a short-lived and very reactive para-benzyne biradical species. It will react with any hydrogen donor such as 1,4-cyclohexadiene which converts to benzene. When quenched by tetrachloromethane the reaction product is a 1,4-dichlorobenzene and with methanol the reaction product is benzyl alcohol.
When the enyne moiety is incorporated into a 10-membered hydrocarbon ring (e.g. cyclodeca-3-ene-1,5-diyne in scheme 2) the reaction, taking advantage of increased ring strain in the reactant, is possible at the much lower temperature of 37 °C.

Naturally occurring compounds such as calicheamicin contain the same 10-membered ring and are found to be cytotoxic. These compounds generate the diradical intermediate described above which can cause single and double stranded DNA cuts. There are novel drugs which attempt to make use of this property, including monoclonal antibodies such as mylotarg.[3]
A biradical mechanism is also proposed for the formation of certain biomolecules found in marine sporolides that have a chlorobenzene unit as part of their structure. In this mechanism a halide salt provides the halogen. A model reaction with the enediyene cyclodeca-1,5-diyn-3-ene, lithium bromide as halogen source and acetic acid as hydrogen source in DMSO at 37 °C supports the theory:[4][5]
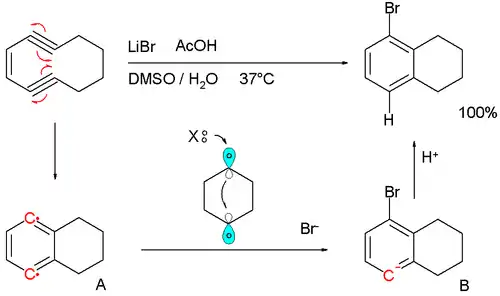
The reaction is found to be first-order in enediyne with the formation of p-benzyne A as the rate-limiting step. The halide ion then donates its two electrons in the formation of a new Br-C bond and radical electron involved is believed to shuttle over a transient C1-C4 bond forming the anion intermediate B. The anion is a powerful base, stripping protons even from DMSO to final product. The dibromide or dihydrogen product (tetralin) never form.
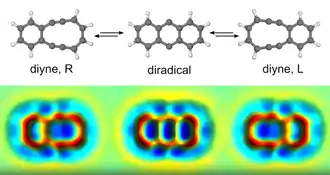
In 2015 IBM scientists demonstrated that a reversible Bergman cyclisation of diyne can be induced by a tip of an atomic force microscope (AFM). They also recorded images of individual diyne molecules during this process.[6] When learning about this direct experimental demonstration Bergman commented, "When we first reported this reaction I had no idea that it would be biologically relevant, or that the reaction could someday be visualized at the molecular level.[7]
References
- Richard R. Jones; Robert G. Bergman (1972). "p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure". J. Am. Chem. Soc. 94 (2): 660–661. doi:10.1021/ja00757a071.
- R. K. Mohamed, P. W. Peterson and I. V. Alabugin; Peterson; Alabugin (2013). "Concerted Reactions that Produce Diradicals andZwitterions: Electronic, Steric, Conformational and Kinetic Control of Cycloaromatization Processes". Chem. Rev. 113 (9): 7089–7129. doi:10.1021/cr4000682. PMID 23600723.
- Luca Banfi; Andrea Basso; Giuseppe Guanti & Renata Riva (2006). "Design and synthesis of heterocycle fused enediyne prodrugs activable at will" (PDF). Arkivoc. HL-1786GR: 261–275.
- Charles L. Perrin; Betsy L. Rodgers & Joseph M. O'Connor (2007). "Nucleophilic Addition to a p-Benzyne Derived from an Enediyne: A New Mechanism for Halide Incorporation into Biomolecules". J. Am. Chem. Soc. 129 (15): 4795–9. doi:10.1021/ja070023e. PMID 17378569.
- Stu Borman (2007). "New Route For Halide Addition". Chemical & Engineering News.
- Schuler, Bruno; Fatayer, Shadi; Mohn, Fabian; Moll, Nikolaj; Pavliček, Niko; Meyer, Gerhard; Peña, Diego; Gross, Leo (25 January 2016). "Reversible Bergman cyclization by atomic manipulation". Nature Chemistry. 8 (3): 220–4. Bibcode:2016NatCh...8..220S. doi:10.1038/nchem.2438. PMID 26892552. S2CID 21611919.
- Sciacca, Chris (25 January 2016). "30 Years of Atomic Force Microscopy: IBM Scientists Trigger and Observe Reactions in an Individual Molecule". Retrieved 25 January 2016.
External links
| Wikimedia Commons has media related to Bergman cyclization. |
- Bergman Cycloaromatization Powerpoint Whitney M. Erwin 2002