Biomolecular condensate
Biomolecular condensates are a class of non-membrane bound organelles and organelle subdomains, specified by physical concepts that date back a long way. As with other organelles, biomolecular condensates are specialized subunits of the cell. However, unlike many organelles, biomolecular condensate composition is not controlled by a bounding membrane. Instead they can form through a range of different processes, the most well-known of which is phase separation of proteins, RNA and other biopolymers into either colloidal emulsions, liquid crystals, solid crystals or aggregates within cells.
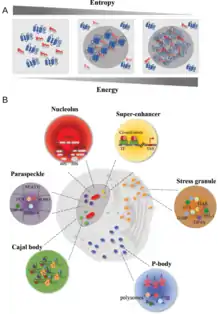
History
Micellar theory
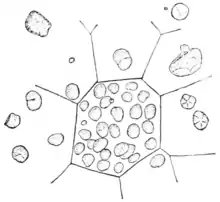
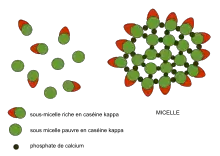
The micellar theory of Carl Nägeli was developed from his detailed study of starch granules in 1858.[1] Amorphous substances such as starch and cellulose were proposed to consist of building blocks, packed in a loosely crystalline array to form which he later termed “micelles”. Water could penetrate between the micelles, and new micelles could form in the interstices between old micelles. The swelling of starch grains and their growth was described by a molecular-aggregate model, which he also applied to the cellulose of the plant cell wall. The modern usage of 'micelle' refers strictly to lipids, but its original usage clearly extended to other types of biomolecule, and this legacy is reflected to this day in the description of milk as being composed of 'casein micelles'.
Colloidal phase separation theory
The term 'colloid' was coined by Wolfgang Ostwald and defined by Thomas Graham in 1861 to describe the behaviour of certain biological macromolecules (starch, albumin, gelatin, etc) and inorganic molecules as slowly diffusing components of cloudy liquid solution-suspensions that were blocked by semi-permeable membranes,[2] while the physics of phase separation was described by Josiah Willard Gibbs in his landmark paper titled On the Equilibrium of Heterogeneous Substances, published in parts between 1875 and 1878.[3] Influenced by Willard Gibbs, important contributions were also made by Johannes Diderik van der Waals, who in 1890 published a treatise on the Theory of Binary Solutions.[4]
.tif.jpg.webp)
The concept of intracellular colloids as an organizing principle for the compartmentalization of living cells dates back to the end of the 19th century, beginning with William Bate Hardy and Edmund Beecher Wilson who described the cytoplasm (then called 'protoplasm') as a colloid.[5][6] Around the same time, Thomas Harrison Montgomery Jr. described the morphology of the nucleolus, an organelle within the nucleus, which has subsequently been shown to form through intracellular phase separation.[7] WB Hardy linked formation of biological colloids with phase separation in his study of globulins, stating that: "The globulin is dispersed in the solvent as particles which are the colloid particles and which are so large as to form an internal phase",[8] and further contributed to the basic physical description of oil-water phase separation.[9]
Colloidal phase separation as a driving force in cellular organisation appealed strongly to Stephane Leduc, who wrote in his influential 1911 book The Mechanism of Life: "Hence the study of life may be best begun by the study of those physico-chemical phenomena which result from the contact of two different liquids. Biology is thus but a branch of the physico-chemistry of liquids; it includes the study of electrolytic and colloidal solutions, and of the molecular forces brought into play by solution, osmosis, diffusion, cohesion, and crystallization." [10]
The primordial soup theory of the origin of life, proposed by Alexander Oparin in Russian in 1924 (published in English in 1936)[11] and by J.B.S. Haldane in 1929,[12] suggested that life was preceded by the formation of what Haldane called a "hot dilute soup" of "colloidal organic substances", and which Oparin referred to as 'coacervates' (after de Jong [13]) - particles composed of two or more colloids which might be protein, lipid or nucleic acid. These ideas strongly influenced the subsequent work of Sidney W. Fox on proteinoid microspheres.
Molecular biology
In 1922, Hermann Staudinger refined the colloidal association theory of Thomas Graham by proposing that certain colloids were composed of high molecular weight polymers (chains linked by covalent bonds), which he termed 'macromolecules' to distinguish them from colloidal clusters of lower molecular weight molecules. So strongly held was colloidal association theory that even Emil Fischer, who had concluded that proteins are polypeptides formed by covalent bonds between amino acid units in 1906, initially refused to believe in the existence of high molecular weight polymers. Staudinger eventually received the Nobel Prize in 1953.[14] Importantly, the discovery of high molecular weight macromolecular polymers cast doubt on the universality of colloidal phase separation in biology, despite the fact that individual polymers could still be observed to associate by clustering and phase separation to form colloids, liquid crystals, solid crystals or aggregates.[15]
A far greater impact occurred when Frederick Sanger determined the amino acid sequence of Insulin[16][17] and Linus Pauling, Robert Corey and Herman Branson correctly proposed the alpha helix and beta sheet as the primary structural motifs in protein secondary structure,[18] while Max Perutz and John Kendrew analysed the 3D structure of myoglobin and later haemoglobin. These breakthroughs in protein structure determination led to a general focus of biologists on atomic-scale amino acid sequence- and 3D conformation-specific protein-protein interactions of a lock and key model type, usually between defined numbers of interacting subunits within a stoichiometric complex. Together with Staudinger's Nobel Prize in 1953[19] this new approach led to a reduced interest in biopolymers as colloids in biology, apart from their propensity to form solid crystals for structure determination by X-ray crystallography.[20][21] Nevertheless, the fields of colloid chemistry and polymer physics continued unabated to characterise the non-stoichiometric interactions occurring during colloidal, liquid crystal and other phase behaviour of macromolecular polymers, particularly synthetic polymers developed for industrial applications.
Support from other disciplines
When cell biologists largely abandoned colloidal phase separation, it was left to relative outsiders - agricultural scientists and physicists - to make further progress in the study of phase separating biomolecules in cells.
Beginning in the early 1970s, Harold M Farrell Jr. at the US Department of Agriculture developed a colloidal phase separation model for milk casein micelles that form within mammary gland cells before secretion as milk.[22]
Also in the 1970s, physicists Tanaka & Benedek at MIT identified phase-separation behaviour of gamma-crystallin proteins from lens epithelial cells and cataracts in solution,[23][24][25][26][27] which Benedek referred to as 'protein condensation'.[28]
_(14763034584).jpg.webp)
In the 1980s and 1990s, Athene Donald's polymer physics lab in Cambridge extensively characterised phase transitions / phase separation of starch granules from the cytoplasm of plant cells, which behave as liquid crystals.[29][30][31][32][33][34][35][36]
In 1991, Pierre-Gilles de Gennes received the Nobel Prize in Physics for developing a generalized theory of phase transitions with particular applications to describing ordering and phase transitions in polymers.[37] Unfortunately, de Gennes wrote in Nature that polymers should be distinguished from other types of colloids, even though they can display similar clustering and phase separation behaviour,[38] a stance that has been reflected in the reduced usage of the term colloid to describe the higher-order association behaviour of biopolymers in modern cell biology and molecular self-assembly.
Phase separation revisited
Advances in confocal microscopy at the end of the 20th century identified proteins, RNA or carbohydrates localising to many non-membrane bound cellular compartments within the cytoplasm or nucleus which were variously referred to as 'puncta/dots',[39][40][41][42] 'signalosomes',[43][44] 'granules',[45] 'bodies', 'assemblies',[42] 'paraspeckles', 'purinosomes',[46] 'inclusions', 'aggregates' or 'factories'. During this time period (1995-2008) the concept of phase separation was re-borrowed from colloidal chemistry & polymer physics and proposed to underlie both cytoplasmic and nuclear compartmentalization.[47][48][49][50][51][52][53][54][55][56]
Since 2009, further evidence for biomacromolecules undergoing intracellular phase transitions (phase separation) has been observed in many different contexts, both within cells and in reconstituted in vitro experiments.[57][58][59][60][61][62][63]
The newly coined term "biomolecular condensate"[64] refers to biological polymers (as opposed to synthetic polymers) that undergo self assembly via clustering to increase the local concentration of the assembling components, and is analogous to the physical definition of condensation.[65][64]
In physics, condensation typically refers to a gas-liquid phase transition.
In biology the term 'condensation' is used much more broadly and can also refer to liquid-liquid phase separation to form colloidal emulsions or liquid crystals within cells, and liquid-solid phase separation to form gels, sols, or suspensions within cells as well as liquid-to-solid phase transitions such as DNA condensation during prophase of the cell cycle or protein condensation of crystallins in cataracts.[66] With this in mind, the term 'biomolecular condensates' was deliberately introduced to reflect this breadth (see below). Since biomolecular condensation generally involves oligomeric or polymeric interactions between an indefinite number of components, it is generally considered distinct from formation of smaller stoichiometric protein complexes with defined numbers of subunits, such as viral capsids or the proteasome - although both are examples of spontaneous molecular self-assembly or self-organisation.
Mechanistically, it appears that the conformational landscape[67] (in particular, whether it is enriched in extended disordered states) and multivalent interactions between intrinsically disordered proteins (including cross-beta polymerisation),[68] and/or protein domains that induce head-to-tail oligomeric or polymeric clustering,[69] might play a role in phase separation of proteins.
Examples
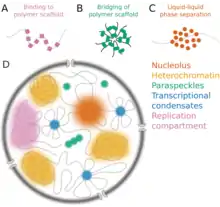
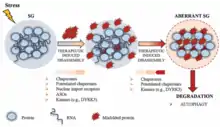
Many examples of biomolecular condensates have been characterized in the cytoplasm and the nucleus that are thought to arise by either liquid-liquid or liquid-solid phase separation.
Cytoplasmic condensates
- Lewy bodies
- Stress granule
- P-body
- Germline P-granules
- Starch granules
- Glycogen granules [70]
- Corneal lens formation and cataracts[71][72][73][26][74][75]
- Other cytoplasmic inclusions such as pigment granules or cytoplasmic crystals
- Purinosomes [46]
- Misfolded protein aggregation such as amyloid fibrils or mutant Haemoglobin S (HbS) fibres in sickle cell disease
- Signalosomes, such as the supramolecular assemblies in the Wnt signaling pathway.[76][77]
- It can also be argued that cytoskeletal filaments form by a polymerisation process similar to phase separation, except ordered into filamentous networks instead of amorphous droplets or granules.
- Bacteria Ribonucleoprotein Bodies (BR-bodies)- In recent studies it has been shown that bacteria RNA degradosomes can assemble into phase‐separated structures, termed bacterial ribonucleoprotein bodies (BR‐bodies), with many analogous properties to eukaryotic processing bodies and stress granules.[78]
- FLOE1 granules: FLOE1 is a prion-like seed-specific protein that controls plant seed germination via phase separation into biomolecular condensates.[79]
Nuclear condensates
Other nuclear structures including heterochromatin and DNA condensation in condensed mitosis chromosomes form by mechanisms similar to phase separation, so can also be classified as biomolecular condensates.
Plasma membrane associated condensates
- Membrane protein, or membrane-associated protein, clustering at neurological synapses, cell-cell tight junctions, or other membrane domains.[81]
Secreted extracellular condensates
- Secreted thyroglobulin colloid and colloid nodules of the Thyroid gland
- Secreted casein ‘micelles’ of the mammary gland
- Serum albumin and globulins
- Secreted lysozyme[82][52]
Lipid enclosed organelles and lipoproteins are not considered condensates
Typical organelles or endosomes enclosed by a lipid bilayer are not considered biomolecular condensates. In addition, lipid droplets are surrounded by a lipid monolayer in the cytoplasm, or in milk, or in tears,[83] so appear to fall under the 'membrane bound' category. Finally, secreted LDL and HDL lipoprotein particles are also enclosed by a lipid monolayer. The formation of these structures involves phase separation to from colloidal micelles or liquid crystal bilayers, but they are not classified as biomolecular condensates, as this term is reserved for non-membrane bound organelles.
Liquid-liquid phase separation (LLPS) in biology
Liquid biomolecular condensates
Liquid-liquid phase separation (LLPS) generates a subtype of colloid known as an emulsion that can coalesce to from large droplets within a liquid. Ordering of molecules during liquid-liquid phase separation can generate liquid crystals rather than emulsions. In cells, LLPS produces a liquid subclass of biomolecular condensate that can behave as either an emulsion or liquid crystal.
The term biomolecular condensates was introduced in the context of intracellular assemblies as a convenient and non-exclusionary term to describe non-stoichiometric assemblies of biomolecules.[64] The choice of language here is specific and important. It has been proposed that many biomolecular condensates form through liquid-liquid phase separation (LLPS) to form colloidal emulsions or liquid crystals in living organisms, as opposed to liquid-solid phase separation to form crystals/aggregates in gels, sols or suspensions within cells or extracellular secretions.[84] However, unequivocally demonstrating that a cellular body forms through liquid-liquid phase separation is challenging,[85][86][87][88] because different material states (liquid vs. gel vs. solid) are not always easy to distinguish in living cells.[89][90] The term "biomolecular condensate" directly addresses this challenge by making no assumption regarding either the physical mechanism through which assembly is achieved, nor the material state of the resulting assembly. Consequently, cellular bodies that form through liquid-liquid phase separation are a subset of biomolecular condensates, as are those where the physical origins of assembly are unknown. Historically, many cellular non-membrane bound compartments identified microscopically fall under the broad umbrella of biomolecular condensates.
In physics, phase separation can be classified into the following types of colloid, of which biomolecular condensates are one example:
| Medium/phase | Dispersed phase | |||
|---|---|---|---|---|
| Gas | Liquid | Solid | ||
| Dispersion medium |
Gas | No such colloids are known. Helium and xenon are known to be immiscible under certain conditions.[91][92] |
Liquid aerosol Examples: fog, clouds, condensation, mist, hair sprays | Solid aerosol Examples: smoke, ice cloud, atmospheric particulate matter |
| Liquid | Foam Example: whipped cream, shaving cream, Gas vesicles | Emulsion or Liquid crystal Examples: milk, mayonnaise, hand cream, latex, biological membranes, micelles, lipoproteins, silk, liquid biomolecular condensates | Sol or suspension Examples: pigmented ink, sediment, precipitates, aggregates, fibres/fibrils/filaments, crystals, solid biomolecular condensates | |
| Solid | Solid foam Examples: aerogel, styrofoam, pumice | Gel Examples: agar, gelatin, jelly, gel-like biomolecular condensates | Solid sol Example: cranberry glass | |
In biology, the most relevant forms of phase separation are either liquid-liquid or liquid-solid, although there have been reports of gas vesicles surrounded by a phase separated protein coat in the cytoplasm of some microorganisms.[93]
Wnt signalling
One of the first discovered examples of a highly dynamic intracellular liquid biomolecular condensate with a clear physiological function were the supramolecular complexes (Wnt signalosomes) formed by components of the Wnt signaling pathway.[54][94][95] The Dishevelled (Dsh or Dvl) protein undergoes clustering in the cytoplasm via its DIX domain, which mediates protein clustering (polymerisation) and phase separation, and is important for signal transduction.[39][40][41][42][44][54] The Dsh protein functions both in planar polarity and Wnt signalling, where it recruits another supramolecular complex (the Axin complex) to Wnt receptors at the plasma membrane. The formation of these Dishevelled and Axin containing droplets is conserved across metazoans, including in Drosophila, Xenopus, and human cells.
P granules
Another example of liquid droplets in cells are the germline P granules in Caenorhabditis elegans.[84][86] These granules separate out from the cytoplasm and form droplets, as oil does from water. Both the granules and the surrounding cytoplasm are liquid in the sense that they flow in response to forces, and two of the granules can coalesce when they come in contact. When (some of) the molecules in the granules are studied (via fluorescence recovery after photobleaching), they are found to rapidly turnover in the droplets, meaning that molecules diffuse into and out of the granules, just as expected in a liquid droplet. The droplets can also grow to be many molecules across (micrometres)[86] Studies of droplets of the Caenorhabditis elegans protein LAF-1 in vitro[96] also show liquid-like behaviour, with an apparent viscosity Pa s. This is about a ten thousand times that of water at room temperature, but it is small enough to enable the LAF-1 droplets to flow like a liquid. Generally, interaction strength (affinity)[97] and valence (number of binding sites)[63] of the phase separating biomolecules influence their condensates viscosity, as well as their overall tendency to phase separate.
References
- Farlow, William G. (1890). "Proceedings of the American Academy of Arts and Sciences". 26. American Academy of Arts & Sciences: 376–381. JSTOR 20013496. Cite journal requires
|journal=(help) - Graham T (December 1861). "Liquid diffusion applied to analysis". Philosophical Transactions of the Royal Society. 151: 183–224. doi:10.1098/rstl.1861.0011.
- Gibbs, J. W. (1961), Scientific Papers, Dover, New York
- Levelt-Sengers JM (2002). "How Fluids Unmix: Discoveries by the School of Van der Waals and Kamerlingh Onnes". R Netherlands Acad of Arts and Sci. Amsterdam.
- Wilson EB (July 1899). "The Structure of Protoplasm". Science. 10 (237): 33–45. Bibcode:1899Sci....10...33W. doi:10.1126/science.10.237.33. PMID 17829686.
- Hardy WB (May 1899). "On the structure of cell protoplasm: Part I. The Structure produced in a Cell by Fixative and Post-mortem change. The Structure of Colloidal matter and the Mechanism of Setting and of Coagulation". The Journal of Physiology. 24 (2): 158–210.1. doi:10.1113/jphysiol.1899.sp000755. PMC 1516635. PMID 16992486.
- Montgomery T (1898). "Comparative cytological studies, with especial regard to the morphology of the nucleolus". Journal of Morphology. 15 (1): 265–582. doi:10.1002/jmor.1050150204. S2CID 84531494.
- Hardy WB (1905). "Colloidal Solution. The Globulins". Journal of Physiology. 33 (4–5): 255–333. doi:10.1113/jphysiol.1905.sp001126. PMC 1465795. PMID 16992817.
- Hardy WB (1912). "The tension of composite fluid surfaces and the mechanical stability of films of fluid". Proceedings of the Royal Society A. 86 (591): 610–635. doi:10.1098/rspa.1912.0053.
- Leduc, Stephane (1911). "The Mechanism of Life".
- Oparin, Alexander. "The Origin of Life" (PDF).
- Haldane, John B. S. "The Origin of Life" (PDF).
- Bungenberg de Jong, H. G., and H. R. Kruyt (1929). "Coacervation (partial miscibility in colloid systems)". Proc Koninklijke Nederlandse Akademie Wetenschappen 32: 849—856
- Nobel Prize in Chemistry 1953.
- Jirgensons, Bruno (1958). "Organic Colloids". Amsterdam-London-New York-Princeton: Elsevier. doi:10.1002/pol.1958.1203312665. Cite journal requires
|journal=(help) - Sanger & Tuppy 1951a; Sanger & Tuppy 1951b; Sanger & Thompson 1953a; Sanger & Thompson 1953b
- Sanger, F. (1958), Nobel lecture: The chemistry of insulin (PDF), Nobelprize.org, retrieved 18 October 2010. Sanger's Nobel lecture was also published in Science: Sanger 1959
- Pauling, L; Corey, RB (1951). "Configurations of Polypeptide Chains With Favored Orientations Around Single Bonds: Two New Pleated Sheets". Proceedings of the National Academy of Sciences of the United States of America. 37 (11): 729–40. Bibcode:1951PNAS...37..729P. doi:10.1073/pnas.37.11.729. PMC 1063460. PMID 16578412.
- Nobel Prize in Chemistry 1953.
- Haas, C.; Drenth, J. (1999). "Understanding protein crystallization on the basis of the phase diagram". Journal of Crystal Growth. 196 (2–4): 388–394. doi:10.1016/S0022-0248(98)00831-8. ISSN 0022-0248.
- Piazza, Roberto (2000). "Interactions and phase transitions in protein solutions". Current Opinion in Colloid & Interface Science. 5 (1–2): 38–43. doi:10.1016/S1359-0294(00)00034-0. ISSN 1359-0294.
- Farrell HM (1973). "Models for Casein Micelle Formation". Journal of Dairy Science. 56 (9): 1195–1206. doi:10.3168/jds.S0022-0302(73)85335-4. PMID 4593735.
- Tanaka T, Benedek GB (Jun 1975). "Observation of protein diffusivity in intact human and bovine lenses with application to cataract". Investigative Ophthalmology & Visual Science. 14 (6): 449–56. PMID 1132941.
- Tanaka T, Ishimoto C, Chylack LT (Sep 1977). "Phase separation of a protein-water mixture in cold cataract in the young rat lens". Science. 197 (4307): 1010–1012. doi:10.1126/science.887936. PMID 887936.
- Ishimoto C, Goalwin PW, Sun ST, Nishio I, Tanaka T (Sep 1979). "Cytoplasmic phase separation in formation of galactosemic cataract in lenses of young rats". Proceedings of the National Academy of Sciences of the United States of America. 76 (9): 4414–4416. doi:10.1073/pnas.76.9.4414. PMC 411585. PMID 16592709.
- Thomson, J. A.; Schurtenberger, P.; Thurston, G. M.; Benedek, G. B. (1987). "Binary liquid phase separation and critical phenomena in a protein/water solution". Proceedings of the National Academy of Sciences. 84 (20): 7079–7083. doi:10.1073/pnas.84.20.7079. ISSN 0027-8424. PMC 299233. PMID 3478681.
- Broide ML, Berland CR, Pande J, Ogun OO, Benedek GB (Jul 1991). "Binary-liquid phase separation of lens protein solutions". Proceedings of the National Academy of Sciences of the United States of America. 88 (13): 5660–4. doi:10.1073/pnas.88.13.5660. PMC 51937. PMID 2062844.
- Benedek GB (September 1997). "Cataract as a protein condensation disease: the Proctor Lecture". Investigative Ophthalmology & Visual Science. 38 (10): 1911–21. PMID 9331254.
- Waigh, T.A.; Gidley, M.J.; Komanshek, B.U.; Donald, A.M. (2000). "The phase transformations in starch during gelatinisation: a liquid crystalline approach". Carbohydrate Research. 328 (2): 165–176. doi:10.1016/s0008-6215(00)00098-7. PMID 11028784.
- Jenkins, P.J.; Donald, A.M. (1998). "Gelatinisation of starch: A combined SAXS/WAXS/DSC and SANS study". Carbohydrate Research. 308 (1–2): 133. doi:10.1016/S0008-6215(98)00079-2.
- Jenkins, P. J.; Donald, A.M. (1995). "The influence of amylose on starch granule structure". International Journal of Biological Macromolecules. 17 (6): 315–21. doi:10.1016/0141-8130(96)81838-1. PMID 8789332.
- Jenkins, P.J.; Cameron, R. E.; Donald, A.M. (1993). "A Universal Feature in the Structure of Starch Granules from Different Botanical Sources". Starch - Stärke. 45 (12): 417. doi:10.1002/star.19930451202.
- Donald, A.M.; Windle, A.H.; Brand, H.R. (1993). "Liquid Crystalline Polymers". Physics Today. 46 (11): 87. Bibcode:1993PhT....46k..87D. doi:10.1063/1.2809100. hdl:2060/19900017655.
- Windle, A.H.; Donald, A.D. (1992). Liquid crystalline polymers. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-30666-9.
- Starch: structure and functionality. Cambridge, England: Royal Society of Chemistry. 1997. ISBN 978-0-85404-742-0.
- The importance of polymer science for biological systems: University of York. Cambridge, England: Royal Society of Chemistry. March 2008. ISBN 978-0-85404-120-6.
- "Press Release: The 1991 Nobel Prize in Physics". Nobelprize.org. Nobel Media AB 2014. Web. 5 May 2017. <https://www.nobelprize.org/nobel_prizes/physics/laureates/1991/press.html>
- de Gennes PG (July 2001). "Ultradivided matter". Nature. 385 (6845): 385. doi:10.1038/35086662. PMID 11473291. S2CID 39983702.
- Cliffe, Adam; Hamada, Fumihiko; Bienz, Mariann (2003). "A Role of Dishevelled in Relocating Axin to the Plasma Membrane during Wingless Signaling". Current Biology. 13 (11): 960–966. doi:10.1016/S0960-9822(03)00370-1. ISSN 0960-9822. PMID 12781135. S2CID 15211115.
- Schwarz-Romond, T. (2005). "The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles". Journal of Cell Science. 118 (22): 5269–5277. doi:10.1242/jcs.02646. ISSN 0021-9533. PMID 16263762. S2CID 16988383.
- Schwarz-Romond, Thomas; Fiedler, Marc; Shibata, Naoki; Butler, P Jonathan G; Kikuchi, Akira; Higuchi, Yoshiki; Bienz, Mariann (2007). "The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization". Nature Structural & Molecular Biology. 14 (6): 484–492. doi:10.1038/nsmb1247. ISSN 1545-9993. PMID 17529994. S2CID 29584068.
- Schwarz-Romond, T.; Metcalfe, C.; Bienz, M. (2007). "Dynamic recruitment of axin by Dishevelled protein assemblies". Journal of Cell Science. 120 (14): 2402–2412. doi:10.1242/jcs.002956. ISSN 0021-9533. PMID 17606995. S2CID 23270805.
- Bilic, J.; Huang, Y.-L.; Davidson, G.; Zimmermann, T.; Cruciat, C.-M.; Bienz, M.; Niehrs, C. (2007). "Wnt Induces LRP6 Signalosomes and Promotes Dishevelled-Dependent LRP6 Phosphorylation". Science. 316 (5831): 1619–1622. doi:10.1126/science.1137065. ISSN 0036-8075. PMID 17569865. S2CID 25980578.
- Bienz, Mariann (2014). "Signalosome assembly by domains undergoing dynamic head-to-tail polymerization". Trends in Biochemical Sciences. 39 (10): 487–495. doi:10.1016/j.tibs.2014.08.006. ISSN 0968-0004. PMID 25239056.
- Kedersha, N.; Anderson, P. (2002). "Stress granules: sites of mRNA triage that regulate mRNA stability and translatability". Biochemical Society Transactions. 30 (6): 963–969. doi:10.1042/bst0300963. ISSN 0300-5127. PMID 12440955.
- An, S.; Kumar, R.; Sheets, E. D.; Benkovic, S. J. (2008). "Reversible Compartmentalization of de Novo Purine Biosynthetic Complexes in Living Cells". Science. 320 (5872): 103–106. doi:10.1126/science.1152241. ISSN 0036-8075. PMID 18388293. S2CID 24119538.
- Walter H, Brooks DE (March 1995). "Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation". FEBS Letters. 361 (2–3): 135–9. doi:10.1016/0014-5793(95)00159-7. PMID 7698310. S2CID 8843457.
- Walter H, Brooks D, Srere P, eds. (October 1999). Microcompartmentation and Phase Separation in Cytoplasm. 192 (1 ed.). Academic Press.
- Brooks, D.E. (1999). "Can Cytoplasm Exist without Undergoing Phase Separation?". Microcompartmentation and Phase Separation in Cytoplasm. International Review of Cytology. 192. pp. 321–330. doi:10.1016/S0074-7696(08)60532-X. ISBN 9780123645968. ISSN 0074-7696. PMID 10610362.
- Walter, Harry (1999). "Consequences of Phase Separation in Cytoplasm". Microcompartmentation and Phase Separation in Cytoplasm. International Review of Cytology. 192. pp. 331–343. doi:10.1016/S0074-7696(08)60533-1. ISBN 9780123645968. ISSN 0074-7696. PMID 10610363.
- Sear, Richard P. (1999). "Phase behavior of a simple model of globular proteins". The Journal of Chemical Physics. 111 (10): 4800–4806. arXiv:cond-mat/9904426. doi:10.1063/1.479243. ISSN 0021-9606. S2CID 15005765.
- Stradner, Anna; Sedgwick, Helen; Cardinaux, Frédéric; Poon, Wilson C. K.; Egelhaaf, Stefan U.; Schurtenberger, Peter (2004). "Equilibrium cluster formation in concentrated protein solutions and colloids" (PDF). Nature. 432 (7016): 492–495. doi:10.1038/nature03109. ISSN 0028-0836. PMID 15565151. S2CID 4373710.
- Iborra FJ (April 2007). "Can visco-elastic phase separation, macromolecular crowding and colloidal physics explain nuclear organisation?". Theoretical Biology & Medical Modelling. 4 (15): 15. doi:10.1186/1742-4682-4-15. PMC 1853075. PMID 17430588.
- Sear, Richard P. (2007). "Dishevelled: a protein that functions in living cells by phase separating". Soft Matter. 3 (6): 680. doi:10.1039/b618126k. ISSN 1744-683X. PMID 32900127.
- Sear, Richard P. (2008). "Phase separation of equilibrium polymers of proteins in living cells". Faraday Discussions. 139: 21–34, discussion 105-28, 419–20. doi:10.1039/b713076g. ISSN 1359-6640. PMID 19048988.
- Dumetz AC, Chockla AM, Kaler EW, Lenhoff AM (Jan 2008). "Protein phase behavior in aqueous solutions: crystallization, liquid-liquid phase separation, gels, and aggregates". Biophysical Journal. 94 (2): 570–83. doi:10.1529/biophysj.107.116152. PMC 2157236. PMID 18160663.
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, et al. (June 2009). "Germline P granules are liquid droplets that localize by controlled dissolution/condensation". Science. 324 (5935): 1729–32. Bibcode:2009Sci...324.1729B. doi:10.1126/science.1172046. PMID 19460965. S2CID 42229928.
- Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, et al. (July 2017). "Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin". Nature. 547 (7662): 236–240. Bibcode:2017Natur.547..236L. doi:10.1038/nature22822. PMC 5606208. PMID 28636604.
- Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, et al. (March 2015). "Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles". Molecular Cell. 57 (5): 936–947. doi:10.1016/j.molcel.2015.01.013. PMC 4352761. PMID 25747659.
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, et al. (August 2015). "A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation". Cell. 162 (5): 1066–77. doi:10.1016/j.cell.2015.07.047. PMID 26317470.
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, et al. (June 2016). "Coexisting Liquid Phases Underlie Nucleolar Subcompartments". Cell. 165 (7): 1686–1697. doi:10.1016/j.cell.2016.04.047. PMC 5127388. PMID 27212236.
- Riback JA, Zhu L, Ferrolino MC, Tolbert M, Mitrea DM, Sanders DW, et al. (2019-10-22). "Composition dependent phase separation underlies directional flux through the nucleolus". bioRxiv: 809210. doi:10.1101/809210.
- Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, et al. (March 2012). "Phase transitions in the assembly of multivalent signalling proteins". Nature. 483 (7389): 336–40. Bibcode:2012Natur.483..336L. doi:10.1038/nature10879. PMC 3343696. PMID 22398450.
- Banani SF, Lee HO, Hyman AA, Rosen MK (May 2017). "Biomolecular condensates: organizers of cellular biochemistry". Nature Reviews. Molecular Cell Biology. 18 (5): 285–298. doi:10.1038/nrm.2017.7. PMC 7434221. PMID 28225081. S2CID 37694361.
- Wheeler RJ, Hyman AA (May 2018). "Controlling compartmentalization by non-membrane-bound organelles". Philosophical Transactions of the Royal Society B: Biological Sciences. 373 (1747): 4666–4684. doi:10.1098/rstb.2017.0193. PMC 5904305. PMID 29632271.
- Benedek GB (September 1997). "Cataract as a protein condensation disease: the Proctor Lecture". Investigative Ophthalmology & Visual Science. 38 (10): 1911–21. PMID 9331254.
- Garaizar, Adiran; Sanchez-Burgos, Ignacio; Collepardo-Guevara, Rosana; Espinosa, Jorge R. (December 2020). "Expansion of Intrinsically Disordered Proteins Increases the Range of Stability of Liquid–Liquid Phase Separation". Molecules. 25 (20): 4705. doi:10.3390/molecules25204705.
- Kato, Masato; McKnight, Steven L. (2017). "Cross-β Polymerization of Low Complexity Sequence Domains". Cold Spring Harbor Perspectives in Biology. 9 (3): a023598. doi:10.1101/cshperspect.a023598. ISSN 1943-0264. PMC 5334260. PMID 27836835.
- Bienz, Mariann (2020). "Head-to-Tail Polymerization in the Assembly of Biomolecular Condensates". Cell. 182 (4): 799–811. doi:10.1016/j.cell.2020.07.037. ISSN 0092-8674.
- Nakano, Akihiro; Trie, Ryozo; Tateishi, Koichi (2014). "Glycogen–Surfactant Complexes: Phase Behavior in a Water/Phytoglycogen/Sodium Dodecyl Sulfate (SDS) System". Bioscience, Biotechnology, and Biochemistry. 61 (12): 2063–2068. doi:10.1271/bbb.61.2063. ISSN 0916-8451. PMID 27396883.
- Tanaka T, Benedek GB (Jun 1975). "Observation of protein diffusivity in intact human and bovine lenses with application to cataract". Investigative Ophthalmology & Visual Science. 14 (6): 449–56. PMID 1132941.
- Tanaka T, Ishimoto C, Chylack LT (Sep 1977). "Phase separation of a protein-water mixture in cold cataract in the young rat lens". Science. 197 (4307): 1010–1012. doi:10.1126/science.887936. PMID 887936.
- Ishimoto C, Goalwin PW, Sun ST, Nishio I, Tanaka T (Sep 1979). "Cytoplasmic phase separation in formation of galactosemic cataract in lenses of young rats". Proceedings of the National Academy of Sciences of the United States of America. 76 (9): 4414–4416. doi:10.1073/pnas.76.9.4414. PMC 411585. PMID 16592709.
- Broide ML, Berland CR, Pande J, Ogun OO, Benedek GB (Jul 1991). "Binary-liquid phase separation of lens protein solutions". Proceedings of the National Academy of Sciences of the United States of America. 88 (13): 5660–4. doi:10.1073/pnas.88.13.5660. PMC 51937. PMID 2062844.
- Benedek GB (September 1997). "Cataract as a protein condensation disease: the Proctor Lecture". Investigative Ophthalmology & Visual Science. 38 (10): 1911–21. PMID 9331254.
- Schaefer KN, Peifer M (February 2019). "Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates". Developmental Cell. 48 (4): 429–444. doi:10.1016/j.devcel.2019.01.025. PMC 6386181. PMID 30782412.
- Gammons M, Bienz M (April 2018). "Multiprotein complexes governing Wnt signal transduction". Current Opinion in Cell Biology. 51 (1): 42–49. doi:10.1016/j.ceb.2017.10.008. PMID 29153704.
- Muthunayake, Nisansala S.; Tomares, D.T; Childers, W. Seth; Schrader, Jared M. (May 2020). "Phase‐separated bacterial ribonucleoprotein bodies organize mRNA decay". WIREs RNA. 11 (3): e1599. doi:10.1002/wrna.1599. PMID 32445438.
- Dorone, Yanniv; Boeynaems, Steven; Jin, Benjamin; Bossi, Flavia; Flores, Eduardo; Lazarus, Elena; Michiels, Emiel; De Decker, Mathias; Baatsen, Pieter; Holehouse, Alex S.; Sukenik, Shahar; Gitler, Aaron D.; Rhee, Seung Y. (August 2020). "Hydration-dependent phase separation of a prion-like protein regulates seed germination during water stress". bioRxiv. doi:10.1101/2020.08.07.242172. S2CID 221096771.
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, et al. (June 2016). "Coexisting Liquid Phases Underlie Nucleolar Subcompartments". Cell. 165 (7): 1686–1697. doi:10.1016/j.cell.2016.04.047. PMC 5127388. PMID 27212236.
- Case, Lindsay B.; Ditlev, Jonathon A.; Rosen, Michael K. (2019). "Regulation of Transmembrane Signaling by Phase Separation". Annual Review of Biophysics. 48 (1): 465–494. doi:10.1146/annurev-biophys-052118-115534. ISSN 1936-122X. PMC 6771929. PMID 30951647.
- Muschol, Martin; Rosenberger, Franz (1997). "Liquid–liquid phase separation in supersaturated lysozyme solutions and associated precipitate formation/crystallization". The Journal of Chemical Physics. 107 (6): 1953–1962. doi:10.1063/1.474547. ISSN 0021-9606.
- Patterson, Matthew; Vogel, Hans J.; Prenner, Elmar J. (2016). "Biophysical characterization of monofilm model systems composed of selected tear film phospholipids". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1858 (2): 403–414. doi:10.1016/j.bbamem.2015.11.025. ISSN 0005-2736. PMID 26657693.
- Tang, Lei (February 2019). "Optogenetic tools light up phase separation". Nature Methods (Paper). 16 (2): 139. doi:10.1038/s41592-019-0310-5. PMID 30700901. S2CID 59525729.(subscription required)
- Hyman AA, Weber CA, Jülicher F (2014-10-11). "Liquid-liquid phase separation in biology". Annual Review of Cell and Developmental Biology. 30 (1): 39–58. doi:10.1146/annurev-cellbio-100913-013325. PMID 25288112.
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, et al. (June 2009). "Germline P granules are liquid droplets that localize by controlled dissolution/condensation". Science. 324 (5935): 1729–32. Bibcode:2009Sci...324.1729B. doi:10.1126/science.1172046. PMID 19460965. S2CID 42229928.
- McSwiggen DT, Mir M, Darzacq X, Tjian R (December 2019). "Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences". Genes & Development. 33 (23–24): 1619–1634. doi:10.1101/gad.331520.119. PMC 6942051. PMID 31594803.
- Posey AE, Holehouse AS, Pappu RV (2018). "Phase Separation of Intrinsically Disordered Proteins". Methods in Enzymology. Elsevier. 611: 1–30. doi:10.1016/bs.mie.2018.09.035. ISBN 978-0-12-815649-0. PMID 30471685.
- Woodruff JB, Hyman AA, Boke E (February 2018). "Organization and Function of Non-dynamic Biomolecular Condensates". Trends in Biochemical Sciences. 43 (2): 81–94. doi:10.1016/j.tibs.2017.11.005. PMID 29258725.
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, et al. (June 2018). "Protein Phase Separation: A New Phase in Cell Biology". Trends in Cell Biology. 28 (6): 420–435. doi:10.1016/j.tcb.2018.02.004. PMC 6034118. PMID 29602697.
- de Swaan Arons, J.; Diepen, G. A. M. (2010). "Immiscibility of gases. The system He-Xe: (Short communication)". Recueil des Travaux Chimiques des Pays-Bas. 82 (8): 806. doi:10.1002/recl.19630820810. ISSN 0165-0513.
- de Swaan Arons, J.; Diepen, G. A. M. (1966). "Gas—Gas Equilibria". J. Chem. Phys. 44 (6): 2322. Bibcode:1966JChPh..44.2322D. doi:10.1063/1.1727043.
- Bayro, Marvin J.; Daviso, Eugenio; Belenky, Marina; Griffin, Robert G.; Herzfeld, Judith (2012). "An Amyloid Organelle, Solid-state NMR Evidence for Cross-β Assembly of Gas Vesicles". Journal of Biological Chemistry. 287 (5): 3479–3484. doi:10.1074/jbc.M111.313049. ISSN 0021-9258. PMC 3271001. PMID 22147705.
- Schaefer KN, Peifer M (February 2019). "Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates". Developmental Cell. 48 (4): 429–444. doi:10.1016/j.devcel.2019.01.025. PMC 6386181. PMID 30782412.
- Gammons M, Bienz M (April 2018). "Multiprotein complexes governing Wnt signal transduction". Current Opinion in Cell Biology. 51 (1): 42–49. doi:10.1016/j.ceb.2017.10.008. PMID 29153704.
- Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC, Eckmann CR, Myong S, Brangwynne CP (June 2015). "The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics". Proceedings of the National Academy of Sciences of the United States of America. 112 (23): 7189–94. Bibcode:2015PNAS..112.7189E. doi:10.1073/pnas.1504822112. PMC 4466716. PMID 26015579.
- Heidenreich M; Georgeson JM; Locatelli E; Rovigatti L; Nandi SK; Steinberg A; Nadav Y; Shimoni E; Safran SA; Doye JPK, Jonathan P. K.; Levy ED (2020-07-13). "Designer protein assemblies with tunable phase diagrams in living cells". Nature Chemical Biology. 16 (9): 939–945. doi:10.1038/s41589-020-0576-z. hdl:11573/1435875. ISSN 1552-4469. PMID 32661377. S2CID 220507058.
Further reading
- Ditlev JA, Case LB, Rosen MK (November 2018). "Who's In and Who's Out-Compositional Control of Biomolecular Condensates". Journal of Molecular Biology. 430 (23): 4666–4684. doi:10.1016/j.jmb.2018.08.003. PMC 6204295. PMID 30099028.
- Banani SF, Lee HO, Hyman AA, Rosen MK (May 2017). "Biomolecular condensates: organizers of cellular biochemistry". Nature Reviews. Molecular Cell Biology. 18 (5): 285–298. doi:10.1038/nrm.2017.7. PMC 7434221. PMID 28225081. S2CID 37694361.
- Hyman AA, Weber CA, Jülicher F (2014). "Liquid-liquid phase separation in biology". Annual Review of Cell and Developmental Biology. 30: 39–58. doi:10.1146/annurev-cellbio-100913-013325. PMID 25288112.
- Dolgin E (March 2018). "What lava lamps and vinaigrette can teach us about cell biology". Nature. 555 (7696): 300–302. Bibcode:2018Natur.555..300D. doi:10.1038/d41586-018-03070-2. PMID 29542707.