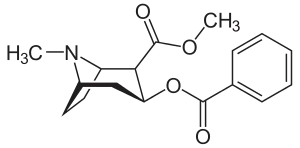
Biosynthesis of cocaine
The biosynthesis of cocaine has long attracted the attention of biochemists and organic chemists. This interest is partly motivated by the strong physiological effects of cocaine, but a further incentive was the unusual bicyclic structure of the molecule. The biosynthesis can be viewed as occurring in two phases, one phase leading to the N-methylpyrrolinium ring, which is preserved in the final product. The second phase incorporates a C4 unit with formation of the bicyclic tropane core.[1]
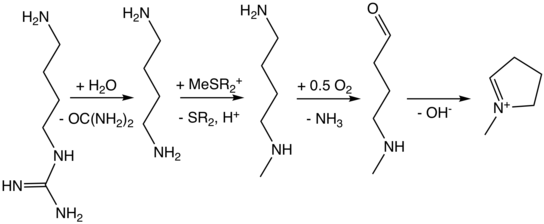
Biosynthesis of N-methyl-pyrrolinium cation
The biosynthesis begins with L-glutamine, which is derived from L-ornithine in plants. The roles of L-ornithine and L-arginine was confirmed by Edward Leete.[2] Ornithine then undergoes a PLP-dependent decarboxylation to form putrescine. In animals, however, the urea cycle derives putrescine from ornithine. L-Ornithine is converted to L-arginine,[3] which is then decarboxylated via PLP to form agmatine. Hydrolysis of the imine derives N-carbamoylputrescine followed with hydrolysis of the urea to form putrescine. The separate pathways of converting ornithine to putrescine in plants and animals have converged. A SAM-dependent N-methylation of putrescine gives the N-methylputrescine, which then undergoes oxidative deamination by the action of diamine oxidase to yield the aminoaldehyde, which spontaneously cyclizes to N-methyl-Δ1-pyrrolinium cation.
Beyond its role in cocaine, the N-methyl-pyrrolinium cation is a precursor to nicotine, hygrine, cuscohygrine, and other natural products.[1]
Conversion of N-methyl-pyrrolinium cation to the tropane
The additional carbon atoms required for the synthesis of cocaine are derived from acetyl-CoA, by addition of two acetyl-CoA units to the N-methyl-Δ1-pyrrolinium cation.[4] The first addition is a Mannich-like reaction with the enolate anion from acetyl-CoA acting as a nucleophile towards the pyrrolinium cation. The second addition occurs through a Claisen condensation. This produces a racemic mixture of the 2-substituted pyrrolidine, with the retention of the thioester from the Claisen condensation. In formation of tropinone from racemic ethyl [2,3-13C2]4(Nmethyl- 2-pyrrolidinyl)-3-oxobutanoate there is no preference for either stereoisomer.[5] In the biosynthesis of cocaine, however, only the (S)-enantiomer can cyclize to form the tropane ring system of cocaine. The stereoselectivity of this reaction was further investigated through study of prochiral methylene hydrogen discrimination.[6] This is due to the extra chiral center at C-2.[7] This process occurs through an oxidation, which regenerates the pyrrolinium cation and formation of an enolate anion, and an intramolecular Mannich reaction. The tropane ring system undergoes hydrolysis, SAM-dependent methylation, and reduction via NADPH for the formation of methylecgonine. The benzoyl moiety required for the formation of the cocaine diester is synthesized from phenylalanine via cinnamic acid.[8] Benzoyl-CoA then combines the two units to form cocaine.

Chemical synthesis
The synthesis and structure elucidation of cocaine was reported by Richard Willstätter in 1898.[9] Willstätter's synthesis derived cocaine from tropinone. Robert Robinson and Edward Leete also made significant contributions.[10]
References
- Leete, Edward (1990). "Recent Developments in the Biosynthesis of the Tropane Alkaloids1". Planta Medica. 56 (4): 339–352. doi:10.1055/s-2006-960979. PMID 2236285.
- Leete E, Marion L, Sspenser ID (October 1954). "Biogenesis of Hyoscyamine". Nature. 174 (4431): 650–1. Bibcode:1954Natur.174..650L. doi:10.1038/174650a0. PMID 13203600.
- Robins, Richard; Waltons, Nicholas; Hamill, John; Parr, Adrian; Rhodes, Michael (1991). "Strategies for the Genetic Manipulation of Alkaloid-Producing Pathways in Plants". Planta Medica. 57 (7 Suppl): S27–S35. doi:10.1055/s-2006-960226. PMID 17226220.
- Dewick, P. M. (2009). Medicinal Natural Products. Chicester: Wiley-Blackwell. ISBN 978-0-470-74276-1.
- R. J. Robins; T. W. Abraham; A. J. Parr; J. Eagles; N. J. Walton (1997). "The Biosynthesis of Tropane Alkaloids in Datura stramonium: The Identity of the Intermediates between N-Methylpyrrolinium Salt and Tropinone". J. Am. Chem. Soc. 119 (45): 10929. doi:10.1021/ja964461p.
- Hoye TR, Bjorklund JA, Koltun DO, Renner MK (January 2000). "N-methylputrescine oxidation during cocaine biosynthesis: study of prochiral methylene hydrogen discrimination using the remote isotope method". Org. Lett. 2 (1): 3–5. doi:10.1021/ol990940s. PMID 10814231.
- E. Leete; J. A. Bjorklund; M. M. Couladis & S. H. Kim (1991). "Late intermediates in the biosynthesis of cocaine: 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoate and methyl ecgonine". J. Am. Chem. Soc. 113 (24): 9286. doi:10.1021/ja00024a039.
- E. Leete; J. A. Bjorklund & S. H. Kim (1988). "The biosynthesis of the benzoyl moiety of cocaine". Phytochemistry. 27 (8): 2553. doi:10.1016/0031-9422(88)87026-2.
- Humphrey AJ, O'Hagan D (October 2001). "Tropane alkaloid biosynthesis. A century old problem unresolved". Nat Prod Rep. 18 (5): 494–502. doi:10.1039/b001713m. PMID 11699882.
- T. Hemscheidt; Vederas, John C. (2000). Leeper, Finian J.; Vederas, John C. (eds.). "Tropane and Related Alkaloids". Top. Curr. Chem. Topics in Current Chemistry. 209: 175. doi:10.1007/3-540-48146-X. ISBN 978-3-540-66573-1.