Putrescine
Putrescine is an organic compound with the formula NH2(CH2)4NH2. A colorless liquid, it is related to cadaverine; both are produced by the breakdown of amino acids. The two compounds are largely responsible for the foul odor of putrefying flesh, but also contribute to the odor of such processes as bad breath and bacterial vaginosis.[1] They are also found in semen and some microalgae, together with related molecules like spermine and spermidine. The polyamines, of which putrescine is one of the simplest, appear to be factors necessary for proper eukaryotic cell division.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butane-1,4-diamine | |
| Other names
1,4-Diaminobutane, 1,4-Butanediamine | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 605282 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.440 |
| EC Number |
|
| 1715 | |
| KEGG | |
| MeSH | Putrescine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2928 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H12N2 | |
| Molar mass | 88.154 g·mol−1 |
| Appearance | Colourless crystals |
| Odor | fishy-ammoniacal, pungent |
| Density | 0.877 g/mL |
| Melting point | 27.5 °C (81.5 °F; 300.6 K) |
| Boiling point | 158.6 °C; 317.4 °F; 431.7 K |
| Miscible | |
| log P | −0.466 |
| Vapor pressure | 2.33 mm Hg at 25 deg C (est) |
Henry's law constant (kH) |
3.54x10−10 atm-cu m/mol at 25 deg C (est) |
Refractive index (nD) |
1.457 |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H228, H302, H312, H314, H331 | |
| P210, P261, P280, P305+351+338, P310 | |
| Flash point | 51 °C (124 °F; 324 K) |
| Explosive limits | 0.98–9.08% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| Related compounds | |
Related alkanamines |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Putrescine and cadaverine were first described in 1885 by the Berlin physician Ludwig Brieger (1849–1919).[2][3][4]
Putrescine is produced on an industrial scale by hydrogenation of succinonitrile.[5] It react with adipic acid to yield the polyamide Nylon 46, which is marketed by DSM under the trade name Stanyl.[6]
Biotechnological production of putrescine from renewable feedstock has been investigated. A metabolically engineered strain of Escherichia coli that produces putrescine at high titer in glucose mineral salts medium has been described.[7]
Biochemistry
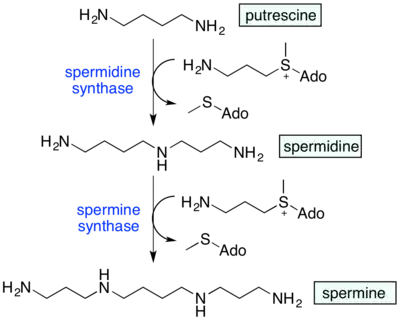
Spermidine synthase uses putrescine and S-adenosylmethioninamine (decarboxylated S-adenosyl methionine) to produce spermidine. Spermidine in turn is combined with another S-adenosylmethioninamine and gets converted to spermine.
Putrescine is synthesized in small quantities by healthy living cells by the action of ornithine decarboxylase.
Putrescine is synthesized biologically via two different pathways, both starting from arginine.
- In one pathway, arginine is converted into agmatine, with a reaction catalyzed by the enzyme arginine decarboxylase (ADC); then agmatine is transformed into N-carbamoylputrescine by agmatine imino hydroxylase (AIH). Finally, N-carbamoylputrescine is converted into putrescine.[8]
- In the second pathway, arginine is converted into ornithine and then ornithine is converted into putrescine by ornithine decarboxylase (ODC).
Toxicity
In rats it has a low acute oral toxicity of 2000 mg/kg body weight, with no-observed-adverse-effect level of 2000 ppm (180 mg/kg body weight/day).[9]
In humans, molecular modelling and docking experiments have shown that putrescine fits into the binding pocket of the human TAAR6 and TAAR8 receptors.[10]
Further reading
- Haglund, William (1996). Forensic taphonomy: The Postmortem Fate of Human Remains. CRC Press. pp. 100. ISBN 0-8493-9434-1.
See also
References
- Yeoman, CJ;Thomas, SM; Miller, ME; Ulanov, AV; Torralba, M; Lucas, S; Gillis, M; Cregger, M; Gomez, A; Ho, M; Leigh, SR; Stumpf, R; Creedon, DJ; Smith, MA; Weisbaum, JS; Nelson, KE; Wilson, BA; White, BA (2013). "A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease". PLOS One. 8 (2): e56111. Bibcode:2013PLoSO...856111Y. doi:10.1371/journal.pone.0056111. PMC 3566083. PMID 23405259.CS1 maint: uses authors parameter (link)
- Brief biography of Ludwig Brieger (in German). Biography of Ludwig Brieger in English.
- Ludwig Brieger, "Weitere Untersuchungen über Ptomaine" [Further investigations into ptomaines] (Berlin, Germany: August Hirschwald, 1885), page 43. From page 43: Ich nenne dasselbe Putrescin, von putresco, faul werden, vermodern, verwesen. (I call this [compound] "putrescine", from [the Latin word] putresco, to become rotten, decay, rot.)
- Ludwig Brieger, "Weitere Untersuchungen über Ptomaine" [Further investigations into ptomaines] (Berlin, Germany: August Hirschwald, 1885), page 39.
- "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry (7th ed.). Retrieved 2007-09-10.
- "Electronic Control Modules (ECU) - Electrical & Electronics - Applications - DSM". Dsm.com. Retrieved 18 December 2015.
- Qian, Zhi-Gang; Xia, Xiao-Xia; Yup Lee, Sang (2009). "Metabolic Engineering of Escherichia coli for the Production of Putrescine: A Four Carbon Diamine". Biotechnology and Bioengineering. doi:10.1002/bit.22502. Archived from the original on 2013-01-05. Retrieved 2010-06-10.
- Srivenugopal KS, Adiga PR (September 1981). "Enzymic conversion of agmatine to putrescine in Lathyrus sativus seedlings. Purification and properties of a multifunctional enzyme (putrescine synthase)". J. Biol. Chem. 256 (18): 9532–41. PMID 6895223.
- Til, H.P.; Falke, H.E.; Prinsen, M.K.; Willems, M.I. (1997). "Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats". Food and Chemical Toxicology. 35 (3–4): 337–348. doi:10.1016/S0278-6915(97)00121-X. ISSN 0278-6915. PMID 9207896.
- Izquierdo, C; Gomez-Tamayo, JC; Nebel, J-C; Pardo, L; Gonzalez, A (2018). "Identifying human diamine sensors for death related putrescine and cadaverine molecules". PLOS Computational Biology. 14 (1): e1005945. Bibcode:2018PLSCB..14E5945I. doi:10.1371/journal.pcbi.1005945. PMC 5783396. PMID 29324768.