Borane carbonyl
Borane carbonyl is the inorganic compound with the formula H3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical interest.[2] It reacts with aqueous base to give boranocarbonate H3BCO22−.[3] Bond distances are B−C, 1.529; C−O, 1.140; 1.194 Å. The H−B−H angle is 113.7°. The CO vibrational band is at 2165 cm−1, 22 cm−1 higher than that of free CO.[4]
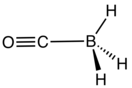 | |
 | |
| Names | |
|---|---|
| IUPAC name
Borane carbonyl | |
| Other names
Carbonyltrihydroboron,Boron, carbonyltrihydro-, (T-4)-; Borane, compd. with carbon monoxide (1:1); Borane-carbon monoxide (1:1); Borine carbonyl (BH3CO); Carbon monoxide-borane; BH3CO; Boron, carbonyltrihydro | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| CH3BO | |
| Molar mass | 41.84 g·mol−1 |
| Appearance | colorless gas |
| Density | 1.71 g/L[1] |
| Melting point | −137[1] °C (−215 °F; 136 K) |
| Boiling point | −64[1] °C (−83 °F; 209 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Experimental data
| INChI | INChIKey | SMILES | IUPAC name | State | Conformation |
|---|---|---|---|---|---|
| InChI=1S/CH3BO/c2-1-3/h2H3 | ZJUVLBOEAXFSHP-UHFFFAOYSA-N | [BH3-][C]=[O+] | 1A1 | C3V |
Enthalpy of vaporization
| ΔvapH (kJ/mol) | Temperature (K) | Reference | Comment |
|---|---|---|---|
| 19.7 | 194 | Stull, 1947 | Based on data from 134. - 209. K. |
Vibrational levels (cm−1)
| Mode Number | Symmetry | Frequency | Comment | |
|---|---|---|---|---|
| Fundamental(cm−1) | Reference | |||
| 1 | A1 | 2380 | webbook | BH3 s-str |
| 2 | A1 | 2165 | webbook | CO str |
| 3 | A1 | 1073 | webbook | BH3 s-deform |
| 4 | A1 | 691 | webbook | B-C str |
| 5 | E | 2444 | webbook | BH3 d-str |
| 6 | E | 1106 | webbook | BH3 d-deform |
| 7 | E | 809 | webbook | BH3 rock |
| 8 | E | 313 | webbook | BCO bend |
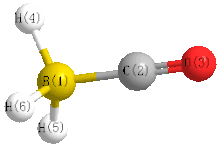
vibrational zero-point energy: 7826.5 cm−1 (from fundamental vibrations)
Rotational Constants (cm−1)
| A | B | C | reference |
|---|---|---|---|
| 1 | 0.28878 | 1977Ven/Tay:17 |
Internal coordinates
distances (r) in Å, angles (a) in degrees, dihedrals (d) in degrees
| Description | Value | unc. | Connectivity | Reference | |||
|---|---|---|---|---|---|---|---|
| Atom 1 | Atom 2 | Atom 3 | Atom 4 | ||||
| rBC | 1.534 | 0.010 | 1 | 2 | 1977Ven/Tay:17 | ||
| rCO | 1.135 | 0.010 | 2 | 3 | 1977Ven/Tay:17 | ||
| rHB | 1.221 | 0.001 | 1 | 4 | 1977Ven/Tay:17 | ||
| aHBC | 103.79 | 0.06 | 2 | 1 | 4 | 1977Ven/Tay:17 | |
| aHBH | 114.5 | 0.15 | 4 | 1 | 5 | 1977Ven/Tay:17 | |
Cartesians
| Atom | x (Å) | y (Å) | z (Å) |
|---|---|---|---|
| B1 | 0.0000 | 0.0000 | -1.3492 |
| C2 | 0.0000 | 0.0000 | 0.1848 |
| O3 | 0.0000 | 0.0000 | 1.3198 |
| H4 | 0.0000 | 1.1858 | -1.6403 |
| H5 | 1.0269 | -0.5929 | -1.6403 |
| H6 | -1.0269 | -0.5929 | -1.6403 |
Atom - Atom Distances
Distances in Å
| B1 | C2 | O3 | H4 | H5 | H6 | |
|---|---|---|---|---|---|---|
| B1 | 1.5340 | 2.6690 | 1.2210 | 1.2210 | 1.2210 | |
| C2 | 1.5340 | 1.1350 | 2.1764 | 2.1764 | 2.1764 | |
| O3 | 2.6690 | 1.1350 | 3.1887 | 3.1887 | 3.1887 | |
| H4 | 1.2210 | 2.1764 | 3.1887 | 2.0539 | 2.0539 | |
| H5 | 1.2210 | 2.1764 | 3.1887 | 2.0539 | 2.0539 | |
| H6 | 1.2210 | 2.1764 | 3.1887 | 2.0539 | 2.0539 |
Experimental Bond Angles
(degrees) from cartesians
| atom1 | atom2 | atom3 | angle | atom1 | atom2 | atom3 | angle |
|---|---|---|---|---|---|---|---|
| B1 | C2 | O3 | 180.000 | C2 | B1 | H4 | 103.790 |
| C2 | B1 | H5 | 103.790 | C2 | B1 | H6 | 103.790 |
| H4 | B1 | H5 | 114.505 | H4 | B1 | H6 | 114.505 |
| H5 | B1 | H6 | 114.505 |
Bond descriptions
Examples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bond
| Bond Type | Count |
|---|---|
| B-C | 1 |
| C#O | 1 |
| H-B | 3 |
Connectivity
| Atom 1 | Atom 2 |
|---|---|
| B1 | C2 |
| B1 | H4 |
| B1 | H5 |
| B1 | H6 |
| C2 | O3 |
Electronic energy levels (cm−1)
| Energy (cm−1) | Degeneracy | reference | description |
|---|---|---|---|
| 0 | 1 | 1A1 |
Ionization Energies (eV)
| Ionization Energy | I.E. unc. | vertical I.E. | v.I.E. unc. | reference |
|---|---|---|---|---|
| 11.140 | 0.020 | 11.920 | 0.020 | webbook |
Electric dipole moment
| State | Config | State description | Conf description | Exp. min. | Dipole (Debye) | Reference | comment | Point Group | Components | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | total | dipole | quadrupole | ||||||||
| 1 | 1 | 1A1 | C3v | True | 1.698 | 1.698 | 1977Ven/Tay:17 | C3v | 1 | 1 | |||
Electric quadrupole moment
| State | Config | State description | Conf description | Exp. min. | Quadrupole (D Å) | Reference | comment | Point Group | Components | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| xx | yy | zz | dipole | quadrupole | ||||||||
| 1 | 1 | 1A1 | C3v | True | C3v | 1 | 1 | |||||
References
| squib | reference | |
|---|---|---|
| webbook | NIST Chemistry Webbook (http://webbook.nist.gov/chemistry) | 10.18434/T4D303 |
References
- www.chemicalbook.com https://www.chemicalbook.com/ChemicalProductProperty_EN_CB72358168.htm. Missing or empty
|title=(help) - Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 165. ISBN 978-0-08-037941-8.
- Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A. P. (2001). "Synthesis and Properties of Boranocarbonate: A Convenient in Situ CO Source for the Aqueous Preparation of [99mTc(OH2)3(CO)3]+". J. Am. Chem. Soc. 123: 3135–3136. doi:10.1021/ja003932b. PMID 11457025.CS1 maint: uses authors parameter (link)
- Jacobsen, H.; Berke, H.; Doering, S.; Kehr, G.; Erker, G.; Froehlich, R.; Meyer, O. (1999). "Lewis Acid Properties of Tris(pentafluorophenyl)borane. Structure and Bonding in L-B(C6F5)3 Complexes". Organometallics. 18: 1724–1735. doi:10.1021/OM981033E.CS1 maint: uses authors parameter (link)
- NIST Chemistry WebBook. "NIST Chemistry WebBook". NIST Chemistry WebBook. Retrieved 25 October 2020.
