Boroxine
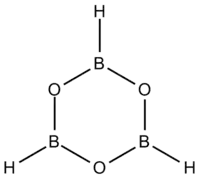
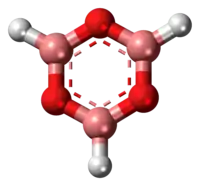
Boroxine (B3H3O3) is a 6-membered, heterocyclic compound composed of alternating oxygen and singly-hydrogenated boron atoms. Boroxine derivatives (boronic anhydrides) such as trimethylboroxine and triphenylboroxine also make up a broader class of compounds called boroxines.[1] These compounds are solids that are usually in equilibrium with their respective boronic acids at room temperature.[1][2][3] Beside being used in theoretical studies, boroxine is primarily used in the production of optics.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,4,6-trihydroxy-1,3,5,2,4,6-trioxatriborinane | |
| Other names
Trihydroxy boroxine, cyclotriboroxane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| B3H3O3 | |
| Molar mass | 83.455 g mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and bonding
Three-coordinate compounds of boron typically exhibit trigonal planar geometry, therefore the boroxine ring is locked in a planar geometry as well.[2][5] These compounds are isoelectronic to benzene. With the vacant p-orbital on the boron atoms, they may possess some aromatic character.[2][6] Boron single-bonds on boroxine compounds are mostly s-character.[5] Ethyl-substituted boroxine has B-O bond lengths of 1.384 Å and B-C bond lengths of 1.565 Å.[6] Phenyl-substituted boroxine has similar bond lengths of 1.386 Å and 1.546 Å respectively, showing that the substituent has little effect on the boroxine ring size.[6]
Substitutions onto a boroxine ring determine its crystal structure. Alkyl-substituted boroxines have the simplest crystal structure. These molecules stack on top of each other, aligning an oxygen atom from one molecule with a boron atom in another, leaving each boron atom between two other oxygen atoms. This forms a tube out of the individual boroxine rings. The intermolecular B-O distance of ethyl-substituted boroxine is 3.462 Å, which is much longer than the B-O bond distance of 1.384 Å. The crystal structure of phenyl-substituted boroxine is more complex. The interaction between the vacant p-orbitals in the boron atoms and the π-electrons in the aromatic, phenyl-substituents cause a different crystal structure. The boroxine ring of one molecule is stacked between two phenyl rings of other molecules. This arrangement allows the phenyl-substituents to donate π-electron density to the vacant boron p-orbitals.[6]
Synthesis
As discovered in the 1930s, boroxines are produced from their corresponding boronic acids by dehydration.[1][2][3] This dehydration can be done either by a drying agent or by heating under a high vacuum.[2] A more recent synthesis of trimethylboroxine involves the reaction of carbon monoxide with borane (B2H6) and lithium borohydride (LiBH4) as a catalyst:[5]
Reactions
Trimethylboroxine is used in the methylation of various aryl halides through palladium-catalyzed Suzuki-Miyaura coupling reactions:[7]
Another form of the Suzuki-Miyaura coupling reaction exhibits selectivity to aryl chlorides:[8]
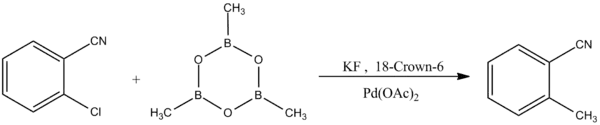 Reaction of Boroxine
Reaction of Boroxine
Boroxines have also been examined as precursors to monomeric oxoborane, HB≡O.[3] This compound quickly converts back to the cyclic boroxine, even at low temperatures.[3]
References
- Brown, H.C. Boranes in Organoc Chemistry; Cornell University Press: Ithaca, 1972; pp. 346–347.
- Hall, Dennis G. (2005). Boronic Acids – Preparation and Applications in Organic Synthesis and Medicine. John Wiley & Sons ISBN 3-527-30991-8.
- Westcott, S.A. (2010). "BO Chemistry Comes Full Circle". Angewandte Chemie International Edition. 49 (48): 9045–9046. doi:10.1002/anie.201003379. PMID 20878818.
- Wu, Q.G.; G. Wu; L. Leon Branca; S. Wang (1999). "B3O3Ph3 (7-Azaindole): Structure, Luminescence, and Fluxionality". Organometallics. 18 (13): 2552–2556. doi:10.1021/om990053t.
- Onak, T. in Organoborane Chemistry; Maitles, P.M., Stone, F.G.A., West, R., Eds.; Academic Press: New York, 1975; pp. 2,4,16,44.
- Haberecht, M.C.; Bolte, Michael; Wagner, Matthias; Lerner, Hans-Wolfram (2005). "A New Polymorph of Tri(p-tolyl)boroxine". Journal of Chemical Crystallography. 35 (9): 657–665. doi:10.1007/s10870-005-3325-y.
- Gray, M.; Andrews, I.P.; Hook, D.F.; Kitteringham, J.; Voyle, M. (2000). "Practical Methylation of Aryl Halides by Suzuki-Miyaura Coupling". Tetrahedron. 41 (32): 6237–6240. doi:10.1016/S0040-4039(00)01038-8.
- Song, C.; Ma, Y.; Chai, Q.; Ma, C.; Jaing, W.; Andrus, M.B. (2005). "Palladium Catalyzed Suzuki-Miyaura Coupling With Aryl Chlorides Using a Bulky Phenanthryl N-heterocyclic Carbene Ligand". Tetrahedron. 61 (31): 7438–7446. doi:10.1016/j.tet.2005.05.071.