Bromate
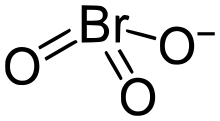
The bromate anion, BrO−
3, is a bromine-based oxoanion. A bromate is a chemical compound that contains this ion. Examples of bromates include sodium bromate, (NaBrO
3), and potassium bromate, (KBrO
3).
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BrO− 3 | |
| Conjugate acid | Bromic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bromates are formed many different ways in municipal drinking water. The most common is the reaction of ozone and bromide:
- Br−
+ O
3 → BrO−
3
Electrochemical processes, such as electrolysis of brine without a membrane operating to form hypochlorite, will also produce bromate when bromide ion is present in the brine solution.
Photoactivation (sunlight exposure) will encourage liquid or gaseous bromine to generate bromate in bromide-containing water.
In laboratories bromates can be synthesized by dissolving Br
2 in a concentrated solution of potassium hydroxide (KOH). The following reactions will take place (via the intermediate creation of hypobromite):
- Br
2 + 2 OH− → Br−
+ BrO−
+ H
2O
- 3 BrO−
→ BrO−
3 + 2 Br−
Human health issues
Bromate in drinking water is undesirable because it is a suspected human carcinogen.[1][2] Its presence in Coca-Cola's Dasani bottled water forced a recall of that product in the UK.[3]
Bromate formation during ozonation
Although few by-products are formed by ozonation, ozone reacts with bromide ions in water to produce bromate. Bromide can be found in sufficient concentrations in fresh water to produce (after ozonation) more than 10 ppb of bromate—the maximum contaminant level established by the USEPA. Proposals to reduce bromate formation include: lowering the water pH below 6.0, limiting the doses of ozone, using an alternate water source with a lower bromide concentration, pretreatment with ammonia, and addition of small concentrations of chloramines prior to ozonation.[4]
Reservoir pollution

On December 14, 2007, the Los Angeles Department of Water and Power (LADWP) announced that it would drain Silver Lake Reservoir and Elysian Reservoir due to bromate contamination. At the Silver Lake and Elysian reservoirs a combination of bromide from well water, chlorine, and sunlight had formed bromate. The decontamination took 4 months, discharging over 600 million US gallons (2.3×106 m3) of contaminated water.[5]
On June 9, 2008 the LADWP began covering the surface of the 10-acre (4 ha), 58-million-US-gallon (0.22×106 m3) open Ivanhoe Reservoir with black, plastic shade balls to block the sunlight which causes the naturally present bromide to react with the chlorine used in treatment. 3 million of the 40 cent balls are required to cover the Ivanhoe and Elysian reservoirs.[6]
Natural occurrence
Currently no bromate-bearing minerals (i.e., the ones with bromate ion being an essential constituent) are known.[7]
References
- "Potassium Bromate (Group 2B)". International Agency for Research on Cancer: Summaries and Evaluations. Canadian Centre for Occupational Health and Safety. Retrieved 2008-03-09.
- Kurokawa, Yuji; Maekawa, A; Takahashi, M; Hayashi, Y (July 1990). "Toxicity and carcinogenicity of potassium bromate—a new renal carcinogen". Environmental Health Perspectives. 87: 309–35. doi:10.1289/EHP.9087309. JSTOR 3431039. PMC 1567851. PMID 2269236.
- "Coke recalls controversial water". BBC News. 2004-03-19. Retrieved 2008-03-09.
- Neemann, Jeff; Hulsey, Robert; Rexing, David; Wert, Eric (2004). "Controlling Bromate Formation: During Ozonation with Chlorine and Ammonia". Journal - American Water Works Association. 96 (2): 26–28. doi:10.1002/j.1551-8833.2004.tb10542.x.
- "DWP To Drain 2 Reservoirs After Potentially Harmful Chemical Found". KNBC News. 2007-12-14. Retrieved 2008-03-09.
- Vara-Orta, Francisco (2008-06-10). "DWP drops 400,000 balls onto Ivanhoe Reservoir". Los Angeles Times.
- "Mindat.org - Mines, Minerals and More". www.mindat.org.