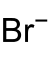Bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group in the Periodic Table.
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Bromide[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3587179 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| 14908 | |||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| Br− | |||
| Molar mass | 79.904 g mol−1 | ||
| Conjugate acid | Hydrogen bromide | ||
| Thermochemistry | |||
Std molar entropy (S |
82 J·mol−1·K−1[2] | ||
Std enthalpy of formation (ΔfH⦵298) |
−121 kJ·mol−1[2] | ||
| Pharmacology | |||
| N05CM11 (WHO) | |||
| Pharmacokinetics: | |||
| 12 d | |||
| Related compounds | |||
Other anions |
Fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Electronic Properties
The bromide ion has an ionic radius of 196 pm.[3] It is the most stable ionic form of bromine; because of its size and small charge, it is highly polarizable.
Natural occurrence
Bromide is present in typical seawater (35 PSU) with a concentration of around 65 mg/L, which is about 0.2% of all dissolved salts. Seafood and deep sea plants generally have higher levels than land-derived foods. Bromargyrite—natural, crystalline silver bromide—is the most common bromide mineral known but is still very rare. In addition to silver, bromine is also in minerals combined with mercury and copper.[4]
Formation of dissolved bromide ion
Like all other halogens, bromine cannot exist in its pure form due to extreme reactivity, and it forms the diatomic molecule Br2. Like gaseous Cl2 and F2, Br2 as a liquid can readily react with water upon being dissolved:
Br2 (l) + H2O (l) → HOBr (aq) + HBr (g)
This forms hypobromous acid (HOBr), and hydrogen bromide (HBr) a colourless gas. The solution is called "bromine water," and its product, HBr gas, can immediately react with water to become hydrobromic acid (HBr(aq)). Like hydrochloric acid (HCl), HBr is a strong acid that readily dissociates by giving off its proton.
HBr(aq) → H+(aq) + Br-(aq)
Since bromine does not occur naturally under atmospheric conditions, the natural pathway for the presence of bromide in the environment is the dissolution of bromide containing minerals. Bromide is highly soluble in water, and this has caused it to accumulate in water bodies.[5]
Extraction of bromine from seawater
Balard and Löwig's method can be used to extract bromine from seawater. First, a seawater sample is tested for the presence of bromide compounds. That same sample is then titrated with aqueous chlorine to produce pure bromine (Br2). The extracted Br2 will then be quantified by measuring its mass or volume. The chemical reaction can be written as:
Cl2 (aq) + 2Br2(aq) —> 2Cl2 + Br2 (l)
Chlorine is a stronger halogen or oxidizing agent than bromine so when the two elements participate in the same chemical reaction, it will act as the oxidizing agent by oxidizing Br [Magazinovic, 2004].
Chemistry
One can test for a bromide ion by adding excess dilute HNO3 followed by dilute aqueous AgNO3 solution. The formation of creamy silver bromide precipitate confirms the existence of bromides.
Medical uses
Bromide compounds, especially potassium bromide, were frequently used as sedatives in the 19th and early 20th centuries. Their use in over-the-counter sedatives and headache remedies (such as Bromo-Seltzer) in the United States extended to 1975 when bromides were withdrawn as ingredients due to chronic toxicity.[6] This use gave the word "bromide" its colloquial connotation of a comforting cliché.[7](e.g. "Children are resilient")
The bromide ion is antiepileptic and as bromide salt, is still used in veterinary medicine in the US. The kidneys excrete bromide ions. The half-life of bromide in the human body (12 days) is long compared with many pharmaceuticals, making dosing challenging to adjust (a new dose may require several months to reach equilibrium). Bromide ion concentrations in the cerebrospinal fluid are about 30% of those in blood and are strongly influenced by the body's chloride intake and metabolism.[8]
Since bromide is still used in veterinary medicine in the United States, veterinary diagnostic labs can routinely measure blood bromide levels. However, this is not a conventional test in human medicine in the US since there are no FDA-approved uses for the bromide. Therapeutic bromide levels are measured in European countries like Germany, where bromide is still used therapeutically in human epilepsy.
Chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption. See potassium bromide.
Lithium bromide was used as a sedative beginning in the early 1900s. However, it fell into disfavour in the 1940s due to the rising popularity of safer and more efficient sedatives (specifically, barbiturates) and when some heart patients died after using a salt substitute (see lithium chloride).[9] Like lithium carbonate and lithium chloride, it was used as a treatment for bipolar disorder.
It has been said that during World War I, British soldiers were given bromide to curb their sexual urges.[10] Lord Dunsany mentions a soldier being given bromide as a sedative for nervous exhaustion and overwork in his play Fame and the Poet (1919).[11]
There are more substantiated reports that bromide was used in the food served at some concentration camps during the Holocaust to both chemically restrain the interned and prevent menstruation in women.[12]
In biology
According to one study, bromine (as bromide) is an essential cofactor in the peroxidising catalysis of sulfonimine crosslinks in collagen IV. This post-translational modification occurs in all animals and bromine is an essential trace element for humans.[13]
Eosinophils need bromide for fighting multicellular parasites. Hypobromite is produced via eosinophil peroxidase, an enzyme that can use chloride but preferentially uses bromide.[14]
Bromide salts are used in hot tubs as mild germicidal agents to generate in situ hypobromite.
Bromide is perhaps a minor necessary nutrient for collagen IV-producing animals in the sea. However, a few sea animals like Murex snails use bromide to make organic compounds. Bromide ion is also heavily concentrated by some ocean algae species to make methyl bromide and other bromo-organic compounds using vanadium Bromo peroxidases.
The average concentration of bromide in human blood in Queensland, Australia, is 5.3±1.4 mg/L and varies with age and gender.[15] Much higher levels may indicate exposure to brominated chemicals. It is also found in seafood.
Further reading
Encyclopedia articles and books
- Christe, K., and S. Schneider (2020), Bromine, Encyclopædia Britannica.
- Emerson, S., and J. Hedges (2011), Chemical Oceanography and the Marine Carbon Cycle, Cambridge University Press, Cambridge.
- Glasow, R. von, and C. Hughes (2014), Biogeochemical Cycles: Bromine, Encyclopedia of Atmospheric Sciences (Second Edition).
- Knight, J., and N. Schlager (2002), Real-life chemistry, Gale Group, Detroit, MI.
- Millero, F. J. (2013), Chemical oceanography, Taylor & Francis, Boca Raton.
- Newton D. E. (2010), Bromine (Revised), Chemical Elements: From Carbon to Krypton.
- Riley, J. P., G. Skirrow, and R. Chester (1975), Chemical Oceanography, Academic Press, London
- Ross, R. (2017), Facts About Bromine, LiveScience.
- Steele, J. H., S. A. Thorpe, and K. K. Turekian (2001), Encyclopedia of Ocean Sciences, Academic Press, San Diego.
- Steele, J. H., S. A. Thorpe, and K. K. Turekian (2009), Encyclopedia of Ocean Sciences, Academic Press, Boston.
- Watkins, T. (2011), Bromine, Environmental Encyclopedia.
Peer-reviewed journal articles for bromine (Br)
- Rattley, M. (2012), Ambiguous bromine, Nature Chemistry, 4(6), 512–512, doi:10.1038/nchem.1361.
- Wisniak, J. (2002), The history of bromine from discovery to commodity, NOPR.
Peer-reviewed journal articles for bromine (Br-)
- Anbar, A. D., Y. L. Yung, and F. P. Chavez (1996), Methyl bromide: Ocean sources, ocean sinks, and climate sensitivity, AGU Journals.
- Foti, S. C., and Naval Ordnance Lab White Oak Md (1972), Concentration of Bromide Ions in Seawater by Isotopic Exchange with Mercurous Bromide, DTIC.
- Gribble, G. W. (2000), The natural production of organobromine compounds, Environmental Science and Pollution Research, 7(1), 37–49, doi:10.1065/espr199910.002.
- Leri A. (2012), The Chemistry of Bromine in Terrestrial and Marine Environments, Science Highlight.
- Magazinovic, R. S., B. C. Nicholson, D. E. Mulcahy, and D. E. Davey (2004), Bromide levels in natural waters: its relationship to levels of both chloride and total dissolved solids and the implications for water treatment, Chemosphere, 57(4), 329–335, doi:10.1016/j.chemosphere.2004.04.056.
- Pilinis, C., D. B. King, and E. S. Saltzman (1996), The oceans: A source or a sink of methyl bromide?, Geophysical Research Letters, 23(8), 817–820, doi:10.1029/96gl00424.
- Stemmler, I., I. Hense, and B. Quack (2015), Marine sources of bromoform in the global open ocean – global patterns and emissions, Biogeosciences, 12(6), 1967–1981, doi:10.5194/bg-12-1967-2015.
- Suzuki, A., Lim, L., Hiroi, T., & Takeuchi, T. (2006, March 20). Rapid determination of bromide in seawater samples by capillary ion chromatography using monolithic silica columns modified with cetyltrimethylammonium ion.
References
- "Bromide – PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. Archived from the original on 2012-11-03.
- Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. ISBN 978-0-618-94690-7.
- Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Crystallographica. (1976). A32, Pages 751-767
- "Mindat.org - Mines, Minerals and More". www.mindat.org. Archived from the original on 2 March 2001. Retrieved 29 April 2018.
- Chemistry of the Elements, N. N. Greenwood, A. Earnshaw, Elsevier, 2012, pp 789
- Adams, Samuel Hopkins (1905). The Great American fraud. Press of the American Medical Association..
- "the definition of bromide". Dictionary.com. Archived from the original on 24 December 2016. Retrieved 21 December 2016.
- Goodman, L. S., and Gilman, A. (eds.) (1970) "Hypnotics and Sedatives", p. 121 in Chapter 10 in The Biological Basis of Therapeutics, Fourth Edition, The MacMillan Co., London.
- Bipolar disorder. webmd.com
- Tanaka, Yuki (2002) Japan's Comfort Women: Sexual slavery and prostitution during World War II and the US Occupation, Routledge, p. 175. ISBN 0415194008.
- Lord Dunsany (August 1919). "Fame and the Poet". The Atlantic Monthly: 175–183. Archived from the original on 2015-12-22.
- Jackson, "The Coming of Age" in Women and the Holocaust, eds Rittter & Roth, p. 80.
- McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG (2014). "Bromine Is an Essential Trace Element for Assembly of Collagen IV Scaffolds in Tissue Development and Architecture". Cell. 157 (6): 1380–92. doi:10.1016/j.cell.2014.05.009. PMC 4144415. PMID 24906154.
- Mayeno, AN; Curran, AJ; Roberts, RL; Foote, CS (1989). "Eosinophils preferentially use bromide to generate halogenating agents". The Journal of Biological Chemistry. 264 (10): 5660–8. PMID 2538427.
- Olszowy, HA; Rossiter, J; Hegarty, J; Geoghegan, P (1998). "Background levels of bromide in human blood". Journal of Analytical Toxicology. 22 (3): 225–30. doi:10.1093/jat/22.3.225. PMID 9602940.