Bromodichloromethane
Bromodichloromethane is a trihalomethane with formula CHBrCl2.
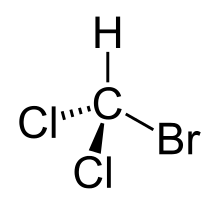 | |
| Names | |
|---|---|
| Preferred IUPAC name
Bromo(dichloro)methane | |
| Other names
Bromodichloromethane Dichlorobromomethane | |
| Identifiers | |
3D model (JSmol) |
|
| 1697005 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.779 |
| EC Number |
|
| 25941 | |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2810 3082 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CHBrCl2 | |
| Molar mass | 163.8 g/mol |
| Appearance | Colorless liquid |
| Density | 1.980 g/cm3 |
| Melting point | −57 °C (−71 °F; 216 K) |
| Boiling point | 90 °C (194 °F; 363 K) |
| 4.5 g/l at 20 °C | |
| -66.3·10−6 cm3/mol | |
Refractive index (nD) |
1.4964 |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
| H302, H315, H319, H335, H350 | |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P304+340, P305+351+338, P308+313, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bromodichloromethane has formerly been used as a flame retardant, and a solvent for fats and waxes and because of its high density for mineral separation. Now it is only used as a reagent or intermediate in organic chemistry.
Bromodichloromethane can also occur in municipally-treated drinking water as a by-product of the chlorine disinfection process.[1]
Notes
- Agency for Toxic Substances & Disease Registry, Accessed 07/10/2012, http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=707&tid=127
External links
- International Chemical Safety Card 0393
- Bromodichloromethane at The Carcinogenic Potency Database
- Toxicological Profile at ATSDR
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.