Cable bacteria
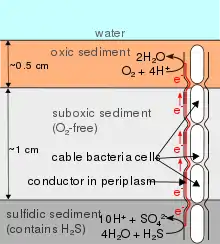
Cable bacteria are filamentous bacteria that conduct electricity across distances over 1 cm in sediment and groundwater aquifers.[1][2] Cable bacteria allow for long distance electron transport, which connects electron donors to electron acceptors, connecting previously separated oxidation and reduction reactions.[3] Cable bacteria couple the reduction of oxygen[2] or nitrate[4] at the sediment's surface to the oxidation of sulfide[2] in the deeper, anoxic, sediment layers.

Discovery
Long-distance electrical conductance in sediment was first observed in 2010 as a spatial separation of sulfide oxidation and oxygen reduction in marine sediment that was interrupted and re-established at a rate faster than could be explained by chemical diffusion.[1] It was later found that this electrical conductance could be observed across a non-conductive layer of glass microspheres, where the only possible conductive structures were filamentous bacteria belonging to the family Desulfobulbaceae.[2] The conductivity of single, live filaments was later demonstrated by observing the oxidation state of cytochromes using Raman microscopy.[5] The same phenomenon was later observed in freshwater sediments[6] and groundwater aquifers.[7] Within a 15 cm thick top layer of sediment, cable bacteria densities providing total length of up to 2 km per square centimeter of surface have been observed.[8]
Morphology
Cable bacteria filaments are 0.4—1.7 µm in diameter and up to 15 mm long.[8] Filaments consist of rod-shaped cells with an average length of 3 µm. Filaments are long strings composed of cells stacked together, and can be as long as 30-70mm. Some filaments are composed of upwards of 10,000 cells.[9] Each cell has between 15 and 54 ridges, and ridges span the entire length of the filament.[2][10] These ridges are hypothesized to contain the cells' conductive structures.[2][11]
Junctions
Cells in a filament are connected by junctions.[12] The diameter of junctions between cells in the filament varies from being smaller than the cell diameter, the same diameter as the cells on either side of the junction, or bulging out to become wider than the cell diameter.[12] Junctions are able to withstand more force without breaking than the cells themselves.[12] Cells on opposite sides of each junction are separated; if one cell bursts, the cell on the other side of the junction will remain intact.[12]
Strings
Cable bacteria contain structures known as strings.[12] Strings are located inside of ridges on the outer membrane and connect one cell to the next.[12] Strings span the length of the entire filament uninterrupted.[12] The width of the strings is about 20-40 nm.[12] The size and function of a string is similar to that of a microtubule.[12] Strings are thought to serve as a structural foundation for filaments and play a key role in maintaining filament shape, especially during growth.[12]
Distribution
Cable bacteria are generally found in reduced sediments.[13] They can be present as a single filament or as an agglomeration of filaments.[13] Cable bacteria have been identified as being intertwined with the root hairs of aquatic plants and are present in the rhizosphere.[13] Their distribution ranges a gradient of salinities; they are present in freshwater, saltwater lakes, and marine habitats.[14][15] Cable bacteria have been identified in a diverse range of climatic conditions worldwide,[16] including Denmark,[2][6] the Netherlands,[11] Japan,[17] Australia,[18] and the United States.[19]
Motility
Cable bacteria lack flagella, but are capable of motility in the form of gliding[20] by propelling themselves forward through the excretion of substances.[21] Cable bacteria have been observed to move as fast as 2.2 µm/s, with an average speed of 0.5 µm/s.[20] Speed of motility in cable bacteria is not related to size of the bacteria.[20] The average distance a cable bacterium glides is approximately 74 µm without interruption.[20] Cable bacteria filaments tend to bend in half, and their movement is led by the apex of the bend as opposed to leading with one tip of the filament.[20] Twisting to move through rotational gliding is rare, but does occur.[20] Cable bacteria likely engage in oxygen chemotaxis, as they are observed to move when in anoxic or hypoxic environments, and cease gliding when contact with oxygen is made.[20] Although motility is important for other microorganisms, once cable bacteria are located in a place that connects oxygen to sulfide, they no longer need to move.[20] The reduced need for motility could explain why the cable bacteria genome contains less operons related to chemotaxis than other Desulfobulbaceae.[21] Less operons related to chemotaxis results in limited motility.[21]
Taxonomy
The two candidate genera of cable bacteria until now described: Electrothrix containing four candidate species, found in marine or brackish sediments, and Electronema containing two candidate species, found in freshwater sediments, seem to be a monophyletic group.[17] Freshwater and marine cable bacteria have been found to be 88% similar based on 16S ribosomal RNA comparisons.[9] These genera are classified within the family Desulfobulbaceae. According to last common ancestor analysis, cable bacteria likely descended from Deltaproteobacteria, Gammaproteobacteria, Chromatiales, and Thiotrichales.[21] Cable bacteria are defined by their function rather than their phylogeny, and it is possible that further cable bacteria taxa will be discovered.
Ecological significance
Cable bacteria strongly influence the geochemical properties of the surrounding environment. Their activity promotes the oxidation of iron at the surface of the sediment, and the resulting iron oxides bind phosphorus-containing compounds[22] and hydrogen sulfide,[23] limiting the amount of phosphorus and hydrogen sulfide in the water. Phosphorus can cause eutrophication, and hydrogen sulfide can be toxic to marine life, meaning that cable bacteria play an important role in maintaining marine ecosystems in coastal areas.
Methane emissions
The presence of cable bacteria can lead to a decrease in methane emissions from saturated soils. The transfer of electrons through cable bacteria allows the sulfate reduction that occurs in inundated soils to be balanced by sulfate oxidation. Oxidation is possible because of the release of electrons through the cable bacteria filaments. Through this balance, sulfate remains readily available for sulfate reducing bacteria, which out compete methanogens. This causes a decrease in production of methane by methanogens.[24]
Practical applications
Cable bacteria have been found associated with benthic microbial fuel cells, devices that convert chemical energy on the ocean floor to electrical energy.[25] In the future, cable bacteria may play a role in increasing the efficiency of microbial fuel cells. Cable bacteria have also been found associated with a bioelectrochemical system for degrading contaminating hydrocarbons in marine sediment [26] and thus may play a role in future oil spill cleanup technologies.
See also
References
- Nielsen LP, Risgaard-Petersen N, Fossing H, Christensen PB, Sayama M (February 2010). "Electric currents couple spatially separated biogeochemical processes in marine sediment". Nature. 463 (7284): 1071–4. Bibcode:2010Natur.463.1071N. doi:10.1038/nature08790. PMID 20182510.
- Pfeffer C, Larsen S, Song J, Dong M, Besenbacher F, Meyer RL, et al. (November 2012). "Filamentous bacteria transport electrons over centimetre distances". Nature. 491 (7423): 218–21. Bibcode:2012Natur.491..218P. doi:10.1038/nature11586. PMID 23103872.
- Nielsen LP, Risgaard-Petersen N (2015). "Rethinking sediment biogeochemistry after the discovery of electric currents". Annual Review of Marine Science. 7: 425–42. Bibcode:2015ARMS....7..425N. doi:10.1146/annurev-marine-010814-015708. PMID 25251266.
- Marzocchi U, Trojan D, Larsen S, Meyer RL, Revsbech NP, Schramm A, et al. (August 2014). "Electric coupling between distant nitrate reduction and sulfide oxidation in marine sediment". The ISME Journal. 8 (8): 1682–90. doi:10.1038/ismej.2014.19. PMC 4817607. PMID 24577351.
- Bjerg JT, Boschker HT, Larsen S, Berry D, Schmid M, Millo D, et al. (May 2018). "Long-distance electron transport in individual, living cable bacteria". Proceedings of the National Academy of Sciences of the United States of America. 115 (22): 5786–5791. doi:10.1073/pnas.1800367115. PMC 5984516. PMID 29735671.
- Risgaard-Petersen N, Kristiansen M, Frederiksen RB, Dittmer AL, Bjerg JT, Trojan D, et al. (September 2015). "Cable Bacteria in Freshwater Sediments". Applied and Environmental Microbiology. 81 (17): 6003–11. doi:10.1128/AEM.01064-15. PMC 4551263. PMID 26116678.
- Müller H, Bosch J, Griebler C, Damgaard LR, Nielsen LP, Lueders T, Meckenstock RU (August 2016). "Long-distance electron transfer by cable bacteria in aquifer sediments". The ISME Journal. 10 (8): 2010–9. doi:10.1038/ismej.2015.250. PMC 4939269. PMID 27058505.
- Schauer R, Risgaard-Petersen N, Kjeldsen KU, Tataru Bjerg JJ, B Jørgensen B, Schramm A, Nielsen LP (June 2014). "Succession of cable bacteria and electric currents in marine sediment". The ISME Journal. 8 (6): 1314–22. doi:10.1038/ismej.2013.239. PMC 4030233. PMID 24451206.
- Meysman FJ (May 2018). "Cable Bacteria Take a New Breath Using Long-Distance Electricity". Trends in Microbiology. 26 (5): 411–422. doi:10.1016/j.tim.2017.10.011. PMID 29174100.
- Cornelissen R, Bøggild A, Thiruvallur Eachambadi R, Koning RI, Kremer A, Hidalgo-Martinez S, et al. (2018). "The Cell Envelope Structure of Cable Bacteria". Frontiers in Microbiology. 9: 3044. doi:10.3389/fmicb.2018.03044. PMC 6307468. PMID 30619135.
- Malkin SY, Rao AM, Seitaj D, Vasquez-Cardenas D, Zetsche EM, Hidalgo-Martinez S, et al. (September 2014). "Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor". The ISME Journal. 8 (9): 1843–54. doi:10.1038/ismej.2014.41. PMC 4139731. PMID 24671086.
- Jiang Z, Zhang S, Klausen LH, Song J, Li Q, Wang Z, et al. (August 2018). "In vitro single-cell dissection revealing the interior structure of cable bacteria". Proceedings of the National Academy of Sciences of the United States of America. 115 (34): 8517–8522. doi:10.1073/pnas.1807562115. PMC 6112711. PMID 30082405.
- Scholz VV, Müller H, Koren K, Nielsen LP, Meckenstock RU (June 2019). "The rhizosphere of aquatic plants is a habitat for cable bacteria". FEMS Microbiology Ecology. 95 (6). doi:10.1093/femsec/fiz062. PMC 6510695. PMID 31054245.
- Trojan D, Schreiber L, Bjerg JT, Bøggild A, Yang T, Kjeldsen KU, Schramm A (July 2016). "A taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema". Systematic and Applied Microbiology. 39 (5): 297–306. doi:10.1016/j.syapm.2016.05.006. PMC 4958695. PMID 27324572.
- Risgaard-Petersen N, Kristiansen M, Frederiksen RB, Dittmer AL, Bjerg JT, Trojan D, et al. (September 2015). Kostka JE (ed.). "Cable Bacteria in Freshwater Sediments". Applied and Environmental Microbiology. 81 (17): 6003–11. doi:10.1128/AEM.01064-15. PMC 4551263. PMID 26116678.
- Burdorf LD, Tramper A, Seitaj D, Meire L, Hidalgo-Martinez S, Zetsche EM, et al. (2017). "Long-distance electron transport occurs globally in marine sediments". Biogeosciences. 14 (3): 683–701. Bibcode:2017BGeo...14..683B. doi:10.5194/bg-14-683-2017.
- Trojan D, Schreiber L, Bjerg JT, Bøggild A, Yang T, Kjeldsen KU, Schramm A (July 2016). "A taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema". Systematic and Applied Microbiology. 39 (5): 297–306. doi:10.1016/j.syapm.2016.05.006. PMC 4958695. PMID 27324572.
- Smith B (December 5, 2014). "Shock as scientists find 'electric' bacteria in the Yarra". The Age.
- Larsen S, Nielsen LP, Schramm A (April 2015). "Cable bacteria associated with long-distance electron transport in New England salt marsh sediment". Environmental Microbiology Reports. 7 (2): 175–9. doi:10.1111/1758-2229.12216. PMID 25224178.
- Bjerg JT, Damgaard LR, Holm SA, Schramm A, Nielsen LP (July 2016). Drake HL (ed.). "Motility of Electric Cable Bacteria". Applied and Environmental Microbiology. 82 (13): 3816–21. doi:10.1128/AEM.01038-16. PMC 4907201. PMID 27084019.
- Kjeldsen KU, Schreiber L, Thorup CA, Boesen T, Bjerg JT, Yang T, et al. (September 2019). "On the evolution and physiology of cable bacteria". Proceedings of the National Academy of Sciences of the United States of America. 116 (38): 19116–19125. doi:10.1073/pnas.1903514116. PMC 6754541. PMID 31427514.
- Sulu-Gambari F, Seitaj D, Meysman FJ, Schauer R, Polerecky L, Slomp CP (February 2016). "Cable Bacteria Control Iron-Phosphorus Dynamics in Sediments of a Coastal Hypoxic Basin". Environmental Science & Technology. 50 (3): 1227–33. Bibcode:2016EnST...50.1227S. doi:10.1021/acs.est.5b04369. PMID 26720721.
- Seitaj D, Schauer R, Sulu-Gambari F, Hidalgo-Martinez S, Malkin SY, Burdorf LD, et al. (October 2015). "Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins". Proceedings of the National Academy of Sciences of the United States of America. 112 (43): 13278–83. Bibcode:2015PNAS..11213278S. doi:10.1073/pnas.1510152112. PMC 4629370. PMID 26446670.
- Scholz VV, Meckenstock RU, Nielsen LP, Risgaard-Petersen N (April 2020). "Cable bacteria reduce methane emissions from rice-vegetated soils". Nature Communications. 11 (1): 1878. doi:10.1038/s41467-020-15812-w. PMC 7171082. PMID 32313021.
- Wendeberg A (January 2010). "Fluorescence in situ hybridization for the identification of environmental microbes". Cold Spring Harbor Protocols. 2010 (1): pdb.prot5366. doi:10.1101/pdb.prot5366. PMID 20150125.
- Matturro B, Cruz Viggi C, Aulenta F, Rossetti S (2017). "Cable Bacteria and the Bioelectrochemical Snorkel: The Natural and Engineered Facets Playing a Role in Hydrocarbons Degradation in Marine Sediments". Frontiers in Microbiology. 8: 952. doi:10.3389/fmicb.2017.00952. PMC 5447156. PMID 28611751.
External links
 Media related to Cable bacteria at Wikimedia Commons
Media related to Cable bacteria at Wikimedia Commons