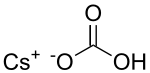
Caesium bicarbonate
Caesium bicarbonate or cesium bicarbonate is a chemical compound with the chemical formula CsHCO3. It can be produced through the following reaction:
- Cs2CO3 + CO2 + H2O → 2 CsHCO3
 | |
| Names | |
|---|---|
| IUPAC name
Caesium bicarbonate | |
| Other names
Cesium bicarbonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.035.943 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CsHCO3 | |
| Molar mass | 193.922 g/mol |
| 67.77 g/100 mL at 20° C | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound can be used for synthesizing caesium salts, but less common than caesium carbonate.
References
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-91. ISBN 0-8493-0462-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.