Cancer-associated fibroblast
A cancer-associated fibroblast (CAF) (also known as tumour-associated fibroblast; carcinogenic- associated fibroblast; activated fibroblast) is a cell type within the tumor microenvironment that promotes tumorigenic features by initiating the remodelling of the extracellular matrix or by secreting cytokines. CAFs are a complex and abundant cell type within the tumour microenvironment;[1] the number cannot decrease, as they are unable to undergo apoptosis.[2]
CAFs have been found to be abundant in a tumour stroma.[3] Myofibroblasts and fibroblasts make up CAFs.[4]
The functions of these CAFs have been known to stimulate angiogenesis, supporting the formation of tumours and thus proliferation of cancer cell and metastasis.[5][6] Cancer cells are usually also drug resistant, which is contributed by CAFs.[3] As such, this interaction is being studied for potential anti-cancer therapy.[7]
Normal fibroblasts aid in the production of components of the extracellular matrix such as collagens, fibres, glycosaminoglycans and glycoproteins and are therefore vital in tissue repair in wound healing.[8]
CAFs however, are derived from either normal fibroblasts, pericytes, smooth muscle cells, fibrocytes or mesenchymal stem cells[9] These CAFs then go on to support tumour growth by secreting growth factors such as Vascular Endothelial Growth Factor (VEGF), Platelet Derived Growth Factor (PDGF) and Fibroblast Growth Factor (FGF) and other chemokines to stimulate angiogenesis and thus the growth of a tumour.[10]
Markers
CAFs produce a number of proteins that are specific to the origin of the cells.[11] However, as there are no specific protein to CAFs, a combination of these proteins are then used as markers to identify CAFs [3] High levels of the marker would mean a low prognosis due to the stage of cancer.
| Name of markers | Functions |
| α-smooth muscle actin (α-SMA) | Marker for myofibroblasts[12] |
| Fibroblast activation protein (FAP) | Marker for myofibroblasts[12] |
| Tenascin-C | Regulates adhesion of cancer cells for invasion[13] |
| Periostin | Product of process of tissue repair[14] |
| Neuron glial antigen-2 (NG2) | More associated with pericytes. What fibroblasts come from |
| Vimentin | Plasma membrane associated protein [11] |
| Desmin | Marker for maturation of microvessels (to mark for angiogenesis)[15] |
| Platelet derived growth factor receptor-α and β (PDGFR α and β) | |
| Fibroblast specific protein-1 (FSP-1)- S100A4 | Myofibroblasts and fibroblasts make up CAFs [4] |
| ASPN | potential new marker of CAF[16] |
| STC1 | Potential new marker of CAF[16] |
Markers for CAFs are notably similar to those of surrounding tumour-associated cells but at the same time, display massive heterogeneity of behaviour, appearance and genotype.[17]
In 2017 Swedish researchers tried to classify molecularly distinct fibroblasts into groups depending on their differential expression of markers. They found overlapping expression patterns which supported the idea that there are transitional states and even identified pluripotency in some patients’ activated fibroblasts (suggesting progenitor cells).
Pleotropic functions (eg. tumour-promoting and tumour-inhibiting) require cell plasticity.[18]
While there are positive markers for CAFs, there are also negative markers namely; cytokeratin and CD3, as CAFS do not have epithelial and endothelial characteristics.[19]
Potential Origin
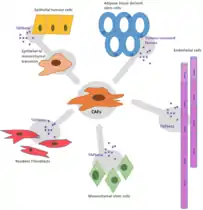
The origins of CAF differ depending on the tumour histotype and where the tumour originated in the first place but can be broadly separated into 4 categories. The origin of each type of CAF has a role in determining the function of that specific cell.[21][22]
Resident
These CAFs arise from fibroblasts within the vicinity of the tumour that have been recruited by cancer derived growth factor. This process is similar to active inflammation with the main difference between these two processes being that, in cancer, the fibroblasts can’t be deactivated which has led to tumours being referred to as “wounds that do not heal.”[23] It is believed that most CAFs arise from differentiated resident fibroblast cells.[24]
The normal fibroblast cells receive a hormone signal from nearby cells, indicating that it must become activated, and is thus classed as a CAF.[2] It is unclear why normal fibroblasts transition into CAFs but it has been found that by adding transforming growth factor- Beta to fibroblasts in culture they start to display features of CAFs.[25] TGF- beta is known to control the activation of fibroblasts in inflammation.
Recruitment from Other Sites
CAFs can also be recruited from a remote source, such as the bone marrow.
Differentiation
CAFs can also be derived from differentiation of other cell types such as MSCs. Another suggested origin is differentiation of endothelial or epithelial cells via trans-differentiation or epithelial to mesenchymal transition, respectively.[26][27]
It has been suggested that CAFs are better conceptualised as a “cell state”[7] Research has found that CAF trans-differentiation can be caused by epigenetic factors.[28]
Roles in Cancer
Prognosis
In general, the presence and density of cancer associated fibroblasts (CAF) point towards a bad prognosis for the patient, and so, are pro-tumour. These could however be used as markers for diagnosis and therapies, thus diagnosing at an earlier stage.
The presence of podoplanin in CAFs has been found to play a fundamental role in worsening the prognosis of patients with lung adenocarcinoma; this could however be helpful as a marker to diagnose at an early stage.[29]
In oesophageal adenocarcinomas, CAFs release the ECM protein periostin and promote tumour cell growth through paracrine signalling. However, blocking specific integrin receptors and pathways can ceases the invasion of tumor cells.[30] The greater the density of CAFs found in oral cancer, the poorer the prognosis, as this significantly decreases the 5 year survival rate. Being female in this study also proved to be a bigger risk factor, with men being protected more against the effects.[31]
Effect on Tumour Cells
Cancer-associated fibroblasts have been found to promote tumour growth. They do this through a number of different mechanisms, notably angiogenesis, metastasis and immune evasion. CAF express various cytokines and factors, which activate and contribute to pathways favouring tumorigenesis.[32] They may disrupt normal cell functions, such as cell cycle regulation and cell death, or signal to specific types of cells to mobilize and activate their pro-tumour actions.[33] Furthermore, it has been found that the effect of CAF on neoplastic cells is unique to the type of tumour cells. Cytokine release from CAFs have been linked to breast carcinomas through the metabolism and production of androgen synthesis enzymes.[34] Furthermore on the topic of the progression of breast cancer, CAFs induces the release growth factors such as FGF and HGF which in turn induces the hyperproliferation of epithelial cells of the breast. EMT and ECM reorganisation are further mechanisms by which the CAFs induce cancer.[35] FSP1, which is secreted by CAFs, promotes tumours through another method - by altering the tumour microenvironment (TME).[36] Some CAFs also recycle the by-products of anaerobic metabolism by resorting to other metabolic pathways to sustain the growth of cancer cells.[37]
Angiogenesis
Angiogenesis is an essential aspect of tumour development. In order for a tumour to grow and significantly increase in size, it must have a sufficient blood supply. If the tumour is unable to develop the blood supply it requires, cells within the tumour will begin to die and further growth will be halted. Angiogenic factors such as vascular endothelial growth factor (VEGF), stromal cell-derived factor 1 (SDF-1), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) are expressed by CAF to encourage the growth of new blood vessels.[3] Some of these factors may also recruit cells that are vital to the angiogenic process, for instance SDF-1 attracts bone-marrow derived endothelial cells.[38]
Metastasis
CAF have been found to promote tumour metastasis in numerous ways. Firstly, they may alter gene expression and have been found to upregulate specific genes involved in pro-tumorigenic pathways including heat shock factor 1 (HSF1).[38] They can also interfere with the function of tumour suppressor genes, such as Tumour protein p53, leading to higher rates of cell proliferation due to the loss of control of the cell cycle.[38] Additionally, CAF have the ability to break down proteins in the extracellular matrix and basement membranes leading to disruption to the normal structure allowing cells to move away from their primary region. The group of proteins known as the matrix metalloproteinases are key to this process.[3] CAF also direct the movement of neoplastic cells by using the Rho-dependent signaling pathway to create tracks for these cells in the matrix.[33]
Chemoresistance
In some cases, the characteristics of CAF provide therapeutic resistance. Soluble factor resistance occurs when CAF either directly secrete signals (cytokines or growth factors) or influence the cells around them to give off similar signals, which reduce the efficacy of therapeutic drugs. For instance, this can either be done by an increased secretion of antiapoptotic factors or by altering the cell environment (e.g. pH) to counteract the actions of the drug.[3] Another form is cell adhesion- mediated drug resistance.[33] This involves the tight attachment of neoplastic cells to the extracellular matrix or stromal cells. For example secretion of TGF-beta allows cancerous cells to bind more successfully to the extracellular matrix thus evading the action of some cancer drugs.
References
- Cirri, Paolo; Chiarugi, Paola (2011-03-12). "Cancer associated fibroblasts: the dark side of the coin". American Journal of Cancer Research. 1 (4): 482–497. ISSN 2156-6976. PMC 3186047. PMID 21984967.
- De Veirman K, Rao L, De Bruyne E, Menu E, Van Valckenborgh E, Van Riet I, Frassanito MA, Di Marzo L, Vacca A, Vanderkerken K (June 2014). "Cancer associated fibroblasts and tumor growth: focus on multiple myeloma". Cancers (Basel). 6 (3): 1363–81. doi:10.3390/cancers6031363. PMC 4190545. PMID 24978438.
- Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H (December 2015). "Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth". Cancers (Basel). 7 (4): 2443–58. doi:10.3390/cancers7040902. PMC 4695902. PMID 26690480.
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (May 2005). "Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion". Cell. 121 (3): 335–48. doi:10.1016/j.cell.2005.02.034. PMID 15882617.
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D (February 2010). "Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner". Cancer Cell. 17 (2): 135–47. doi:10.1016/j.ccr.2010.04.018. PMID 20138012.
- Giannoni, Elisa; Bianchini, Francesca; Masieri, Lorenzo; Serni, Sergio; Torre, Eugenio; Calorini, Lido; Chiarugi, Paola (2010-08-31). "Reciprocal Activation of Prostate Cancer Cells and Cancer-Associated Fibroblasts Stimulates Epithelial-Mesenchymal Transition and Cancer Stemness". Cancer Research. 70 (17): 6945–6956. doi:10.1158/0008-5472.can-10-0785. PMID 20699369.
- Madar S, Goldstein I, Rotter V (August 2013). "'Cancer associated fibroblasts'--more than meets the eye". Trends Mol Med. 19 (8): 447–53. doi:10.1016/j.molmed.2013.05.004. PMID 23769623.
- Tracy, Lauren E.; Minasian, Raquel A.; Caterson, E.j. (2014-08-20). "Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound". Advances in Wound Care. 5 (3): 119–136. doi:10.1089/wound.2014.0561. ISSN 2162-1918. PMC 4779293. PMID 26989578.
- Räsänen, Kati; Vaheri, Antti (2010). "Activation of fibroblasts in cancer stroma". Experimental Cell Research. 316 (17): 2713–2722. doi:10.1016/j.yexcr.2010.04.032. PMID 20451516.
- Weber, Cynthia E.; Kuo, Paul C. (2012). "The tumor microenvironment". Surgical Oncology. 21 (3): 172–177. doi:10.1016/j.suronc.2011.09.001. PMID 21963199.
- Augsten M (2014). "Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment". Front Oncol. 4: 62. doi:10.3389/fonc.2014.00062. ISSN 2234-943X. PMC 3973916. PMID 24734219.
- Sappino AP, Skalli O, Jackson B, Schürch W, Gabbiani G (May 1988). "Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues". Int. J. Cancer. 41 (5): 707–12. doi:10.1002/ijc.2910410512. ISSN 1097-0215. PMID 2835323.
- Orend G, Chiquet-Ehrismann R (December 2006). "Tenascin-C induced signaling in cancer". Cancer Lett. 244 (2): 143–63. doi:10.1016/j.canlet.2006.02.017. PMID 16632194.
- Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, Kii I, Horie H, Nagai H, Kudo A, Fukayama M (August 2008). "Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon". J. Histochem. Cytochem. 56 (8): 753–64. doi:10.1369/jhc.2008.951061. PMC 2443605. PMID 18443362.
- Arentz G, Chataway T, Price TJ, Izwan Z, Hardi G, Cummins AG, Hardingham JE (December 2011). "Desmin expression in colorectal cancer stroma correlates with advanced stage disease and marks angiogenic microvessels". Clin Proteomics. 8 (1): 16. doi:10.1186/1559-0275-8-16. ISSN 1559-0275. PMC 3259060. PMID 22141345.
- Orr B, Riddick AC, Stewart GD, Anderson RA, Franco OE, Hayward SW, Thomson AA (March 2012). "Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate". Oncogene. 31 (9): 1130–42. doi:10.1038/onc.2011.312. ISSN 0950-9232. PMC 3307063. PMID 21804603.
- Baglole, Carolyn J.; Smith, Terry J.; Foster, David; Sime, Patricia J.; Feldon, Steve; Phipps, Richard P. (2006). Tissue Repair, Contraction and the Myofibroblast. Biotechnology Intelligence Unit. Springer, Boston, MA. pp. 32–39. doi:10.1007/0-387-33650-8_4. ISBN 9780387336497.
- Busch S, Andersson D, Bom E, Walsh C, Ståhlberg A, Landberg G (April 2017). "Cellular organization and molecular differentiation model of breast cancer-associated fibroblasts". Mol. Cancer. 16 (1): 73. doi:10.1186/s12943-017-0642-7. ISSN 1476-4598. PMC 5376683. PMID 28372546.
- Sukowati CH, Anfuso B, Crocé LS, Tiribelli C (March 2015). "The role of multipotent cancer associated fibroblasts in hepatocarcinogenesis". BMC Cancer. 15: 188. doi:10.1186/s12885-015-1196-y. ISSN 1471-2407. PMC 4389787. PMID 25879842.
- Calon A, Tauriello DV, Batlle E (April 2014). "TGF-beta in CAF-mediated tumor growth and metastasis". Semin. Cancer Biol. 25: 15–22. doi:10.1016/j.semcancer.2013.12.008. PMID 24412104.
- Cirri, Paolo; Chiarugi, Paola (2011-03-12). "Cancer associated fibroblasts: the dark side of the coin". American Journal of Cancer Research. 1 (4): 482–497. ISSN 2156-6976. PMC 3186047. PMID 21984967.
- Nair N, Calle AS, Zahra MH, Prieto-Vila M, Oo AK, Hurley L, Vaidyanath A, Seno A, Masuda J, Iwasaki Y, Tanaka H, Kasai T, Seno M (July 2017). "A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment". Sci Rep. 7 (1): 6838. Bibcode:2017NatSR...7.6838N. doi:10.1038/s41598-017-07144-5. ISSN 2045-2322. PMC 5533745. PMID 28754894.
- Dvorak, Harold F. (1986-12-25). "Tumors: Wounds That Do Not Heal". New England Journal of Medicine. 315 (26): 1650–1659. doi:10.1056/nejm198612253152606. ISSN 0028-4793. PMID 3537791.
- Wang, Jingyi; Min, Anjie; Gao, Shan; Tang, Zhangui (2014-05-01). "Genetic regulation and potentially therapeutic application of cancer-associated fibroblasts in oral cancer". Journal of Oral Pathology & Medicine. 43 (5): 323–334. doi:10.1111/jop.12098. ISSN 1600-0714. PMID 23782231.
- Kalluri R, Zeisberg M (May 2006). "Fibroblasts in cancer". Nat. Rev. Cancer. 6 (5): 392–401. doi:10.1038/nrc1877. PMID 16572188.
- Karnoub, Antoine E.; Dash, Ajeeta B.; Vo, Annie P.; Sullivan, Andrew; Brooks, Mary W.; Bell, George W.; Richardson, Andrea L.; Polyak, Kornelia; Tubo, Ross (2007). "Mesenchymal stem cells within tumour stroma promote breast cancer metastasis". Nature. 449 (7162): 557–563. Bibcode:2007Natur.449..557K. doi:10.1038/nature06188. PMID 17914389.
- Dov., Zipori (2009). Biology of stem cells and the molecular basis of the stem state. Totowa, N.J.: Humana. ISBN 9781607611295. OCLC 432708883.
- Rudnick JA, Kuperwasser C (October 2012). "Stromal biomarkers in breast cancer development and progression". Clin. Exp. Metastasis. 29 (7): 663–72. doi:10.1007/s10585-012-9499-8. ISSN 0262-0898. PMID 22684404.
- Kubouchi Y, Yurugi Y, Wakahara M, Sakabe T, Haruki T, Nosaka K, Miwa K, Araki K, Taniguchi Y, Shiomi T, Nakamura H, Umekita Y (February 2018). "Podoplanin expression in cancer-associated fibroblasts predicts unfavourable prognosis in patients with pathological stage IA lung adenocarcinoma" (PDF). Histopathology. 72 (3): 490–499. doi:10.1111/his.13390. ISSN 1365-2559. PMID 28881047.
- Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, Thirdborough S, Mead A, Clemons N, Mellone M, Uzoho C, Primrose JN, Blaydes JP, Thomas GJ (February 2015). "Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma". J. Pathol. 235 (3): 466–77. doi:10.1002/path.4467. ISSN 1096-9896. PMC 4312957. PMID 25345775.
- VERED, M.; GRAIZEL, D.; ZLOTOGORSKI-HURVITZ, A.; ROSEN, E.; TSESIS, I. (2017). "The Prognostic Significance of Cancer-Associated Fibroblasts in Oral Cancer: A Systematic Review of the Literature and Meta-Analysis". Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 124 (3): e222. doi:10.1016/j.oooo.2017.06.080.
- Gascard P, Tlsty TD (May 2016). "Carcinoma-associated fibroblasts: orchestrating the composition of malignancy". Genes Dev. 30 (9): 1002–19. doi:10.1101/gad.279737.116. ISSN 0890-9369. PMC 4863733. PMID 27151975.
- Öhlund D, Elyada E, Tuveson D (July 2014). "Fibroblast heterogeneity in the cancer wound". J. Exp. Med. 211 (8): 1503–23. doi:10.1084/jem.20140692. ISSN 0022-1007. PMC 4113948. PMID 25071162.
- Kikuchi K, McNamara KM, Miki Y, Moon JY, Choi MH, Omata F, Sakurai M, Onodera Y, Rai Y, Ohi Y, Sagara Y, Miyashita M, Ishida T, Ohuchi N, Sasano H (December 2017). "Effects of cytokines derived from cancer-associated fibroblasts on androgen synthetic enzymes in estrogen receptor-negative breast carcinoma". Breast Cancer Res. Treat. 166 (3): 709–723. doi:10.1007/s10549-017-4464-5. ISSN 0167-6806. PMID 28831645.
- Buchsbaum, Rachel J.; Oh, Sun Young (2016-01-27). "Breast Cancer-Associated Fibroblasts: Where We Are and Where We Need to Go". Cancers. 8 (2): 19. doi:10.3390/cancers8020019. PMC 4773742. PMID 26828520.
- Xing, Fei; Saidou, Jamila; Watabe, Kounosuke (2010-01-01). "Cancer associated fibroblasts (CAFs) in tumor microenvironment". Frontiers in Bioscience. 15: 166–179. doi:10.2741/3613. ISSN 1093-9946. PMC 2905156. PMID 20036813.
- Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E (January 2006). "Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma". Cancer Res. 66 (2): 632–7. doi:10.1158/0008-5472.CAN-05-3260. PMID 16423989.
- Kalluri R (August 2016). "The biology and function of fibroblasts in cancer". Nat. Rev. Cancer. 16 (9): 582–98. doi:10.1038/nrc.2016.73. PMID 27550820.