Cannabidiolic acid
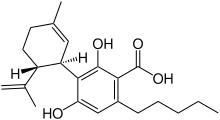
Cannabidiolic acid, also known as CBDA, is a cannabinoid found in cannabis plants.[1] It is most abundant in the glandular trichomes on the female seedless flowers or more accurately infructescence often colloquially referred to as buds.[2] CBDA is the chemical precursor to cannabidiol (CBD). Through the process of decarboxylation cannabidol is derived via a loss of a carbon and two oxygen atoms from the 1 positon of the benzoic acid ring.
 | |
| Names | |
|---|---|
| IUPAC name
2,4-Dihydroxy-3-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-6-pentylbenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H30O4 | |
| Molar mass | 358.478 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemical composition
Cannabidiolic acid is biosynthesized by Cannabidiolic acid synthase from the conjugation of olivetolic acid and cannabigerolic acid[3]
References
- Takeda, Shuso (2013). "[Medicinal chemistry and pharmacology focused on cannabidiol, a major component of the fiber-type cannabis]". Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan. 133 (10): 1093–1101. doi:10.1248/yakushi.13-00196. ISSN 1347-5231. PMID 24088353.
- Livingston, Samuel J.; Quilichini, Teagen D.; Booth, Judith K.; Wong, Darren C. J.; Rensing, Kim H.; Laflamme‐Yonkman, Jessica; Castellarin, Simone D.; Bohlmann, Joerg; Page, Jonathan E.; Samuels, A. Lacey (2020). "Cannabis glandular trichomes alter morphology and metabolite content during flower maturation". The Plant Journal. n/a (n/a): 37–56. doi:10.1111/tpj.14516. ISSN 1365-313X. PMID 31469934.
- PubChem. "Cannabidiolic acid". pubchem.ncbi.nlm.nih.gov. Retrieved 2019-12-23.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
