Carbon dioxide (data page)
This page provides supplementary chemical data on carbon dioxide.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as SIRI, and follow its directions. MSDS for solid carbon dioxide is available from Pacific Dry Ice, inc.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.000449 at 589.3 nm and 0 °C [1] |
| Dielectric constant, εr | 1.60 ε0 at 0 °C, 50 atm |
| Average energy per C=O bond | 804.4 kJ/mol at 298 K (25 °C)[2] |
| Bond length | C=O 116.21 pm (1.1621 Å) [3] |
| Bond angle | O–C–O: 180°[3] |
| Magnetic susceptibility | ? |
| Surface tension | 4.34 dyn/cm at 20 °C and equilibrium pressure |
| Viscosity[4] of liquid at equilibrium pressure |
0.0925 mPa·s at 5 °C 0.0852 mPa·s at 10 °C 0.0712 mPa·s at 20 °C 0.0625 mPa·s at 25 °C 0.0321 mPa·s at 31.1 °C |
Thermodynamic properties
| Phase behavior | |
|---|---|
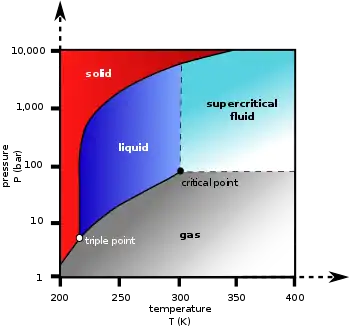
| Triple point | 216.58 K (−56.57 °C), 518.5 kPa |
| Critical point | 304.18 K (31.03 °C), 7.38 MPa |
| Std enthalpy change of fusion, ΔfusH |
8.647 kJ/mol at triple point[5] |
| Entropy change of fusion, ΔfusS |
40 J/(mol·K) at triple point |
| Std enthalpy change of vaporization,[6] ΔvapH |
15.326 kJ/mol at 215.7 K (−57.5 °C) |
| Std entropy change of vaporization, ΔvapS |
70.8 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
−427.4 kJ/mol |
| Standard molar entropy,[7] S |
51.07 J/(mol·K) |
| Heat capacity,[7] cp |
2.534 J/(mol·K) at 15.52 K (−257.63 °C) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol·K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−393.52 kJ/mol |
| Standard molar entropy, S |
213.79 J/(mol·K) |
| Heat capacity,[8] cp |
33.89 J/(mol K) at –75 °C |
| Heat capacity ratio[8] γ = cp/cv |
1.37 at –75 °C |
| van der Waals' constants[9] | a = 363.96 L2 kPa/mol2 b = 0.04267 liter per mole |
| Equilibrium with carbon monoxide[10] CO + ½O2 → CO2 K = pK = log10 K |
pK = 45.0438 at T = 298.16 K |
Solubility in water at various temperatures
| Aqueous Solubility of CO2 at 101.3 kPa (1 atm) partial pressure[11] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- ‡Second column of table indicates solubility at each given temperature in volume of CO2 as it would be measured at 101.3 kPa and 0 °C per volume of water.
- The solubility is given for "pure water", i.e., water which contain only CO2. This water is going to be acidic. For example, at 25 °C the pH of 3.9 is expected (see carbonic acid). At less acidic pH values, the solubility will increase because of the pH-dependent speciation of CO2.
Vapor pressure of solid and liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| P in atm (2sf, derived from mm Hg) | 0.0013 | 0.013 | 0.053 | 0.13 | 0.53 | 1.0 | 2.0 | 5.0 | 10 | 20 | 40 | 60 | |
| P in kPa (derived from mm Hg / atm) | 0.13 | 1.3 | 5.3 | 13 | 53 | 101.325 | 202.65 | 506.625 | 1013.25 | 2026.5 | 4053 | 6079.5 | |
| T in °C | −134.3(s) | −119.5(s) | −108.6(s) | −100.2(s) | −85.7(s) | −78.2(s) | −69.1(s) | −56.7 | −39.5 | −18.9 | 5.9 | 22.4 | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. Annotation "(s)" indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. For kPa values, where datum is whole numbers of atmospheres exact kPa values are given, elsewhere 2 significant figures derived from mm Hg data.
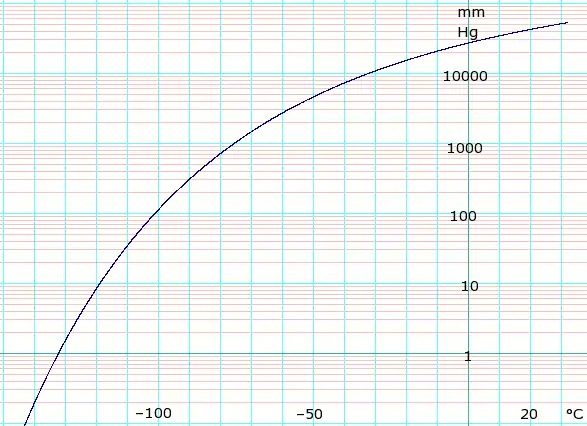
Phase diagram
Liquid/vapor equilibrium thermodynamic data
The table below gives thermodynamic data of liquid CO2 in equilibrium with its vapor at various temperatures. Heat content data, heat of vaporization, and entropy values are relative to the liquid state at 0 °C temperature and 3483 kPa pressure. To convert heat values to joules per mole values, multiply by 44.095 g/mol. To convert densities to moles per liter, multiply by 22.678 cm3 mol/(L·g). Data obtained from CRC Handbook of Chemistry and Physics, 44th ed. pages 2560–2561, except for critical temperature line (31.1 °C) and temperatures −30 °C and below, which are taken from Lange's Handbook of Chemistry, 10th ed. page 1463.
| Carbon dioxide liquid/vapor equilibrium thermodynamic data | ||||||||
| Temp. °C | Pvap Vapor pressure kPa | Hliq Heat content liquid J/g | Hvap Heat content vapor J/g | ΔvapH Heat of vapor- ization J/g | ρvap Density of vapor g/cm3 | ρliq Density of liquid g/cm3 | Sliq Entropy liquid J/mol-°C | Svap Entropy vapor J/mol-°C |
|---|---|---|---|---|---|---|---|---|
| −56.6 | 518.3 | 1.179 | ||||||
| −56.0 | 531.8 | 1.177 | ||||||
| −54.0 | 579.1 | 1.169 | ||||||
| −52.0 | 629.6 | 1.162 | ||||||
| −50.0 | 683.4 | 1.155 | ||||||
| −48.0 | 740.6 | 1.147 | ||||||
| −46.0 | 801.3 | 1.139 | ||||||
| −44.0 | 865.6 | 1.131 | ||||||
| −42.0 | 933.8 | 1.124 | ||||||
| −40.0 | 1005.7 | 1.116 | ||||||
| −38.0 | 1081.9 | 1.108 | ||||||
| −36.0 | 1161.8 | 1.100 | ||||||
| −34.0 | 1246.2 | 1.092 | ||||||
| −32.0 | 1335.1 | 1.084 | ||||||
| −30.0 | 1428.6 | 1.075 | ||||||
| −28.89 | 1521 | −55.69 | 237.1 | 292.9 | 0.03846 | 1.0306 | −9.48 | 43.41 |
| −27.78 | 1575 | −53.76 | 237.3 | 291.0 | 0.03987 | 1.0276 | −9.13 | 43.21 |
| −26.67 | 1630 | −51.84 | 237.6 | 289.4 | 0.04133 | 1.0242 | −8.78 | 43.01 |
| −25.56 | 1686 | −49.87 | 237.6 | 287.5 | 0.04283 | 1.0209 | −8.45 | 42.78 |
| −24.44 | 1744 | −47.91 | 237.8 | 285.7 | 0.04440 | 1.0170 | −8.10 | 42.56 |
| −23.33 | 1804 | −45.94 | 237.8 | 283.6 | 0.04600 | 1.0132 | −7.75 | 42.36 |
| −22.22 | 1866 | −43.93 | 237.8 | 281.7 | 0.04767 | 1.0093 | −7.40 | 42.14 |
| −21.11 | 1928 | −41.92 | 237.8 | 279.6 | 0.04938 | 1.0053 | −7.05 | 41.94 |
| −20.00 | 1993 | −39.91 | 237.8 | 277.8 | 0.05116 | 1.0011 | −6.68 | 41.71 |
| −18.89 | 2059 | −37.86 | 237.8 | 275.7 | 0.05300 | 0.9968 | −6.31 | 41.49 |
| −17.78 | 2114 | −35.82 | 237.6 | 273.6 | 0.05489 | 0.9923 | −5.98 | 41.27 |
| −16.67 | 2197 | −33.73 | 237.6 | 271.2 | 0.05686 | 0.9875 | −5.61 | 41.05 |
| −15.56 | 2269 | −31.64 | 237.3 | 269.2 | 0.05888 | 0.9829 | −5.26 | 40.83 |
| −14.44 | 2343 | −29.54 | 237.3 | 266.9 | 0.06098 | 0.9782 | −4.91 | 40.61 |
| −13.33 | 2418 | −27.41 | 237.1 | 264.5 | 0.06314 | 0.9734 | −4.54 | 40.39 |
| −12.22 | 2495 | −25.27 | 236.9 | 262.2 | 0.06539 | 0.9665 | −4.17 | 40.15 |
| −11.11 | 2574 | −23.09 | 236.7 | 259.7 | 0.06771 | 0.9639 | −3.80 | 39.92 |
| −10.00 | 2654 | −20.90 | 236.4 | 257.3 | 0.07011 | 0.9592 | −3.43 | 39.68 |
| −8.89 | 2738 | −18.69 | 235.9 | 254.8 | 0.07259 | 0.9543 | −3.06 | 39.46 |
| −7.78 | 2823 | −16.45 | 235.7 | 252.2 | 0.07516 | 0.9494 | −2.69 | 39.22 |
| −6.67 | 2910 | −14.18 | 235.2 | 249.4 | 0.07783 | 0.9443 | −2.32 | 38.98 |
| −5.56 | 2999 | −11.90 | 234.8 | 246.6 | 0.08059 | 0.9393 | −1.94 | 38.74 |
| −4.44 | 3090 | −9.977 | 234.3 | 243.8 | 0.08347 | 0.9340 | −1.57 | 38.50 |
| −3.89 | 3136 | −8.410 | 234.1 | 242.4 | 0.08494 | 0.9313 | −1.37 | 38.37 |
| −2.78 | 3230 | −6.046 | 233.6 | 239.7 | 0.08797 | 0.9260 | −0.98 | 38.12 |
| −1.67 | 3327 | −3.648 | 232.9 | 236.6 | 0.09111 | 0.9206 | −0.59 | 37.88 |
| −0.56 | 3425 | −1.222 | 232.4 | 233.6 | 0.09438 | 0.9150 | −0.20 | 37.62 |
| 0.56 | 3526 | 1.234 | 231.7 | 230.5 | 0.09776 | 0.9094 | 0.20 | 37.36 |
| 1.67 | 3629 | 3.728 | 231.0 | 227.3 | 0.1013 | 0.9036 | 0.61 | 37.08 |
| 2.78 | 3735 | 6.268 | 230.4 | 224.0 | 0.1050 | 0.8975 | 1.01 | 36.83 |
| 3.89 | 3843 | 8.445 | 229.4 | 220.5 | 0.1088 | 0.8914 | 1.42 | 36.55 |
| 5.00 | 3953 | 11.46 | 228.5 | 217.0 | 0.1128 | 0.8850 | 1.83 | 36.25 |
| 6.11 | 4067 | 14.13 | 227.6 | 213.4 | 0.1169 | 0.8784 | 2.25 | 35.98 |
| 7.22 | 4182 | 16.85 | 226.5 | 209.7 | 0.1213 | 0.8716 | 2.69 | 35.68 |
| 8.33 | 4300 | 19.63 | 225.4 | 205.8 | 0.1258 | 0.8645 | 3.12 | 35.39 |
| 9.44 | 4420 | 22.46 | 224.3 | 201.8 | 0.1306 | 0.8571 | 3.56 | 35.07 |
| 10.56 | 4544 | 25.36 | 223.1 | 197.7 | 0.1355 | 0.8496 | 4.02 | 34.76 |
| 11.67 | 4670 | 28.33 | 221.8 | 193.4 | 0.1408 | 0.8418 | 4.48 | 34.45 |
| 12.78 | 4798 | 31.35 | 220.3 | 188.9 | 0.1463 | 0.8338 | 4.94 | 34.11 |
| 13.89 | 4929 | 34.49 | 218.8 | 184.3 | 0.1521 | 0.8254 | 5.42 | 33.76 |
| 15.00 | 5063 | 37.30 | 217.2 | 179.5 | 0.1583 | 0.8168 | 5.92 | 33.41 |
| 16.11 | 5200 | 41.03 | 215.1 | 174.4 | 0.1648 | 0.8076 | 6.42 | 33.02 |
| 17.22 | 5340 | 44.48 | 213.6 | 169.1 | 0.1717 | 0.7977 | 6.96 | 32.66 |
| 18.33 | 5482 | 48.03 | 211.5 | 163.5 | 0.1791 | 0.7871 | 7.49 | 32.25 |
| 19.44 | 5628 | 51.71 | 209.4 | 157.6 | 0.1869 | 0.7759 | 8.04 | 31.83 |
| 20.56 | 5776 | 55.61 | 207.0 | 151.4 | 0.1956 | 0.7639 | 8.63 | 31.38 |
| 21.67 | 5928 | 59.66 | 204.3 | 144.7 | 0.2054 | 0.7508 | 9.24 | 30.90 |
| 22.78 | 6083 | 63.97 | 201.5 | 137.5 | 0.2151 | 0.7367 | 9.89 | 30.39 |
| 23.89 | 6240 | 68.58 | 198.4 | 129.8 | 0.2263 | 0.7216 | 10.57 | 29.85 |
| 25.00 | 6401 | 73.51 | 194.8 | 121.3 | 0.2387 | 0.7058 | 11.31 | 29.24 |
| 26.11 | 6565 | 78.91 | 190.7 | 111.8 | 0.2532 | 0.6894 | 12.10 | 28.60 |
| 27.22 | 6733 | 84.94 | 186.0 | 101.1 | 0.2707 | 0.6720 | 12.99 | 27.84 |
| 28.33 | 6902 | 91.88 | 180.4 | 88.49 | 0.2923 | 0.6507 | 14.00 | 26.95 |
| 29.44 | 7081 | 100.4 | 173.1 | 72.72 | 0.3204 | 0.6209 | 15.24 | 25.85 |
| 30.00 | 7164 | 105.6 | 168.4 | 62.76 | 0.3378 | 0.5992 | 16.01 | 25.15 |
| 30.56 | 7253 | 112.3 | 162.3 | 50.04 | 0.3581 | 0.5661 | 16.99 | 24.24 |
| 31.1 | 7391 | 0.00 | 0.4641 | 0.4641 | ||||
| Temp. °C | Pvap Vapor pressure kPa | Hliq Heat content liquid J/g | Hvap Heat content vapor J/g | ΔvapH Heat of vapor- ization J/g | ρvap Density of vapor g/cm3 | ρliq Density of liquid g/cm3 | Sliq Entropy liquid J/mol-°C | Svap Entropy vapor J/mol-°C |
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR[lower-alpha 1] | |
| Major absorption bands | 2350 and 667 cm−1
(4.25 and 14.99 um) |
| NMR | |
| Proton NMR | not applicable |
| Carbon-13 NMR | 125.0[12] |
| MS | |
| Masses of main fragments | |
Notes
- Because nitrogen and oxygen are symmetrical and carbon dioxide and water vapor are not, the air in an infrared spectrophotometer may show absorbances for CO2 and water. This is easily overcome by subtracting a blank spectrum from the experimental spectrum, and instruments are often purged with dry nitrogen as well.
References
- "Refractive index of gases". NPL. Archived from the original on 7 October 2010. Retrieved 7 April 2010.
- Darwent, B. deB. (1970). "Bond Dissociation Energies in Simple Molecules" Nat. Stand. Ref. Data Ser., Nat. Bur. Stand. (U.S.) 31, 52 pages.
- "CCCBDB listing of experimental data page 2". cccbdb.nist.gov. Retrieved 1 December 2018.
- Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- "Gas Encyclopaedia". Air Liquide. Retrieved 1 June 2007.
- "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 8 May 2007.
- W. F. Giauque and C. J. Egan, "Carbon Dioxide. The Heat Capacity and Vapor Pressure of the Solid. The Heat of Sublimation. Thermodynamic and Spectroscopic Values of the Entropy", Journal of Chemical Physics, vol. 5, pp. 45–54, 1937.
- Lange's Handbook of Chemistry, 10th ed, pp. 1525–1528.
- Lange's Handbook of Chemistry, 10th ed, pp. 1522–1524.
- Lange's Handbook of Chemistry, 10th ed. pp. 1573–1576.
- Lange's Handbook of Chemistry, 10th ed., p 1100
- Reich, H. J. "C-13 Chemical Shifts". Organic Chem Info. University of Wisconsin. Archived from the original on 2 March 2015. Retrieved 31 May 2015.
- Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.